
Physiology News Magazine
Transmission of cortical oscillations to motoneuron output for force control
The precise control of muscles for movement execution requires reliable transmission of information in the central nervous system. At the spinal motoneurons, information transmission is partly linear because the input is common to a large number of neurons. This generates coherence between the EEG and EMG signals, a measure whose validity is, however, debated.
Features
Transmission of cortical oscillations to motoneuron output for force control
The precise control of muscles for movement execution requires reliable transmission of information in the central nervous system. At the spinal motoneurons, information transmission is partly linear because the input is common to a large number of neurons. This generates coherence between the EEG and EMG signals, a measure whose validity is, however, debated.
Features
Francesco Negro and Dario Farina
Department of Neurorehabilitation Engineering, Bernstein Center for Computational Neuroscience,
University Medical Center Göttingen, Georg-August University, Göttingen, Germany
https://doi.org/10.36866/pn.84.35


The motor cortex controls the generation of voluntary movements through the direct and indirect activation of spinal motoneurons. Direct corticospinal projections on alpha-motoneurons have been demonstrated by histological findings (Lemon, 2008) and electrophysiological measures (Conway et al. 1995; Halliday et al. 1998). Moreover, the observation that the motor cortex activity recorded by the magnetoencephalogram (MEG) and the electroencephalogram (EEG) is coherent with the surface electromyogram (EMG) detected over the active muscle indicates that the cortical command is transmitted to the spinal motoneurons in a partly linear way (Fig. 1). Despite this observation which has been confirmed in several independent studies and various conditions, its explanation is not obvious.
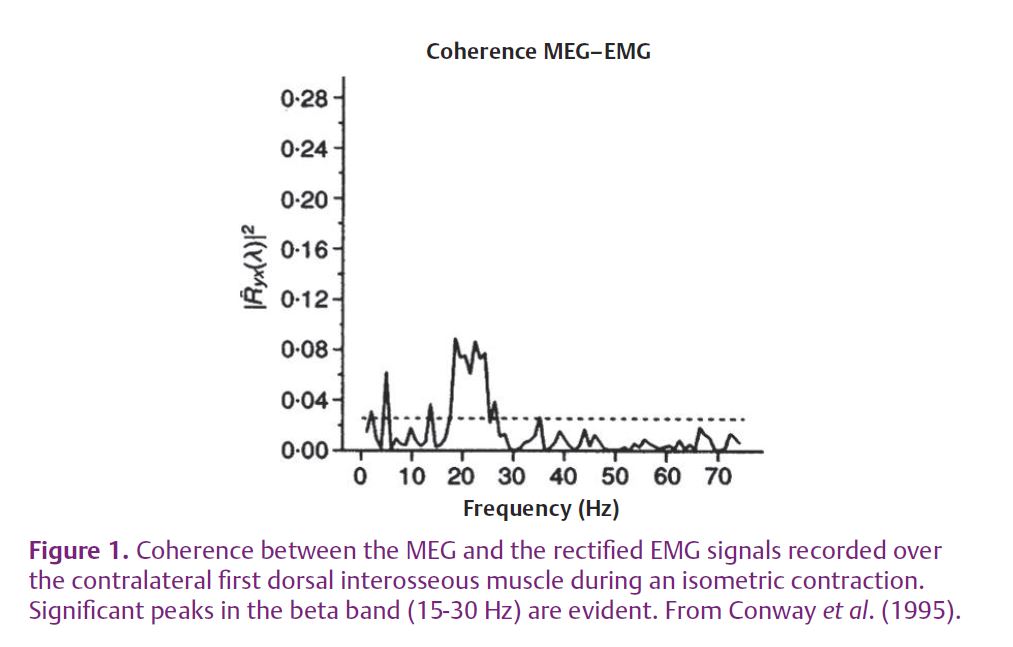
The neurons, which are the main components of the central nervous system, are non-linear systems. Therefore, why is the coherence between EEG and EMG significant in certain frequency bands? Coherence analysis is indeed a linear tool that indicates linear correlation in the frequency domain between two signals.
Because of the intrinsic properties of neurons, the input to a neuron is not directly proportional to the generated output, but it depends on several parameters, such as the discharge statistics. Moreover, if several inputs are integrated together, as for motoneurons, the resulting output is not, in general, the summation of the individual responses. For such reasons, the control of neural systems based on the tuning of individual inputs seems an impractical problem. However, since population coding has been demonstrated to play a key role in the decoding of external stimuli (and thus in the transmission of information) in several regions of the central nervous system (Averbeck et al. 2006), it is likely that it also plays a key role in the control of voluntary movements.
Spinal motoneurons discharge at relatively low frequency during moderate muscle contractions. Therefore, since the motoneuron behaves essentially as an integrate-and-sample process, any input signal with fast variability is under-sampled by each individual motoneuron. However, if the input is common to a population of motoneurons, the actual sampling rate is increased by a factor that is associated with the number of motoneurons in the population and to their discharge rates. Moreover, the noise which is not common to all motoneurons is attenuated in the cumulative output spike train.
We have recently investigated the corticospinal transmission using EEG and single motor unit recordings in humans during isometric low force contractions (Negro & Farina, 2011). Starting from an analytical derivation previously proposed for an integrate-and-fire neuron (Nakao et al. 1997), it was demonstrated that under the assumption of sinusoidal inputs, the output of the motoneuron pool reproduced the input with greater fidelity (greater linearity) when the number of motoneurons that received the common input increased. Moreover, the level of interference created by the spiking processes was, at least partly, overcome by integrating the input with a relatively small number of motoneurons. The experimental results confirmed the analytical findings, showing that the significant level of coherence between the EEG activity recorded over the motor cortex and the motor unit spike trains in the beta band increases with the number of spike trains used in the calculation but that after summing more than a few output spike trains, it reaches a plateau value (Fig 2). These results completed previous observations on low-frequency oscillations of motoneuron spike trains (De Luca et al. 1982; Negro et al. 2009) and demonstrated that the input to motoneurons is largely spread across the entire pool.
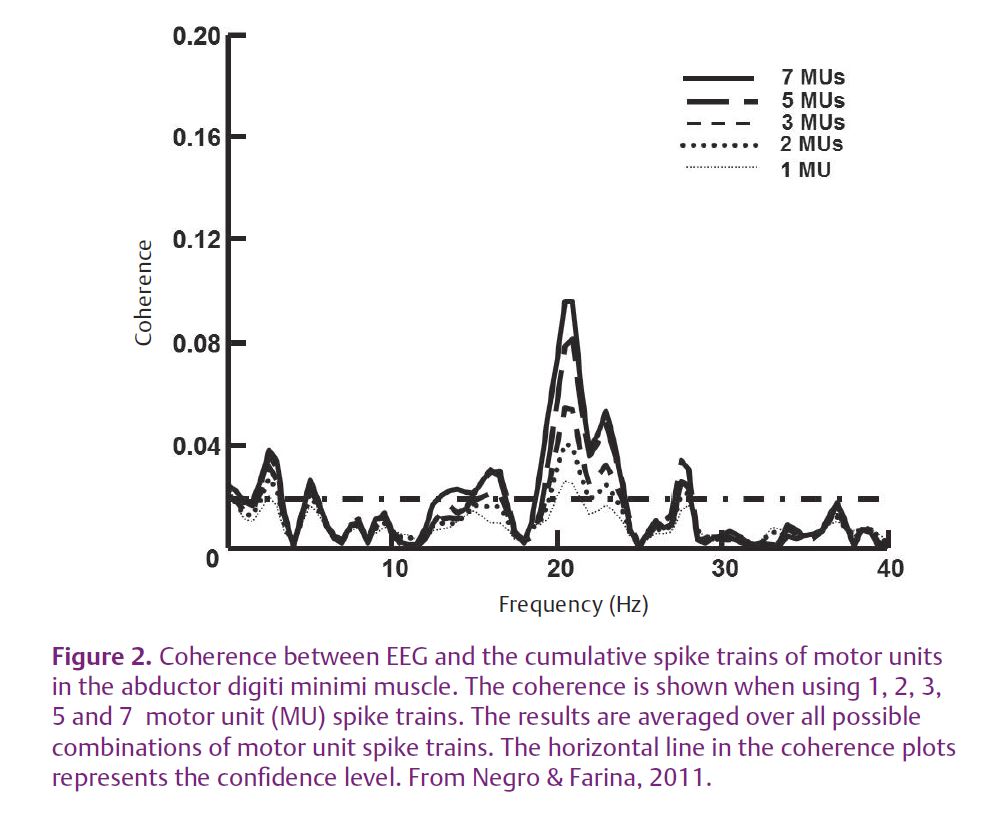
These results, however, leave some open questions. For example, it is not clear why the cortical oscillations in the mu/alpha band are usually not found to be coherent with motoneuron activity, contrary to oscillations in the beta band. Recently a potential contribution of spinal circuits and out-of-phase afferent feedbacks have been pointed out to account for this observation (Williams & Baker, 2009). Moreover, it has recently been shown in simulation that the presence of other common inputs to motoneurons, independent of the cortical input, could decorrelate the output, which is another way of stating that afferent input common to the motoneurons can decrease or cancel the coherence of cortical input with motoneuron output (Negro, 2011).
Beside the discussion on which frequency bands are represented in the EEG–EMG coherence, one fundamental question remains unanswered: why are oscillations in the alpha and beta band transmitted to the motoneuron pool if most of their power is reduced anyway by the low-pass filtering effect of the muscle dynamics (Baldissera et al. 1998)? For example, in a recent study, we have shown that the frequency content below 2 Hz in the cumulative spike train of motoneurons accounts for more than 60% of the total variance of the generated joint force whereas higher frequencies in the spike trains had negligible influence on force (Negro et al. 2009). The functional significance of oscillations at greater frequencies transmitted to the neural drive to muscles is thus unclear. Several hypotheses could be discussed; however, the simplest answer may be that these oscillations are only a by-product of the spiking nature of the neurons and are filtered out by the muscle contraction to perform a stable and precise movement. This hypothesis is partly in agreement with the observed beta, but not alpha, band coherence. Since the cut-off frequency of the muscle contraction is indeed at around 3–5 Hz, it is necessary to delete the presence of oscillations in the alpha band with some spinal interaction (Williams & Baker, 2009) because these oscillations would not be completely removed by the muscle dynamics. It is interesting to note in this respect that alpha oscillations correspond to pathological tremor frequencies (Elble & Randall, 1976 ), so that it can be hypothesized that pathological tremor corresponds to the inability of neural mechanisms (such as afferent pathways) to suppress such oscillations. On the other hand, the beta band is completely attenuated by the muscle dynamics and thus it is not necessary for the system to attenuate it with neural mechanisms, which may explain the reason why it remains in the neural drive to muscle and is observable in the EEG–EMG coherence analysis.
In conclusion, there is evidence that oscillations present at the input of motoneurons can be transmitted partly linearly to the output if the input is common to the motoneurons. This explains the presence of coherence between EEG and EMG as well as between EEG and cumulative motor unit spike trains. Nevertheless, the actual functional significance of the observed linear association between cortical oscillations at relatively high frequencies and the neural drive to muscles is unclear due to the filtering of the neural drive by the muscle dynamics. We propose here the hypothesis that these oscillations may be a by-product of the transmission of information from different parts of the central nervous system. This hypothesis, which is purely speculative, needs to be proven (or disproven) in further experiments.
References
Averbeck BB, Latham PE & Pouget A (2006). Neural correlations, population coding and computation. Nat Rev Neurosci 7, 358–366.
Baldissera F, Cavallari P & Cerri G (1998). Motoneuronal pre-compensation for the low-pass filter characteristics of muscle. A quantitative appraisal in cat muscle units. J Physiol 511, 611–627.
Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI & Rosenberg JR (1995). Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489, 917–924.
De Luca CJ, LeFever RS, McCue MP & Xenakis AP (1982). Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329, 129–142.
Elble RJ & Randall JE (1976). Motor-unit activity responsible for 8 to 12 Hz component of physiological finger tremor. J Neurophysiol 39, 370–383.
Halliday DM, Conway BA, Farmer SF & Rosenberg JR (1998). Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett 1, 5–8.
Lemon RN (2008). Descending pathways in motor control. Annu Rev Neurosci 31, 195–218.
Nakao M, Norimatsu M, Mizutani Y & Yamamoto M (1997). Spectral distortion properties of the integral pulse frequency modulation model. IEEE Trans Biomed Eng 44, 419–426.
Negro F & Farina D (2011). Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589, 629–637. http://jp.physoc.org/content/589/3/629.long
Negro F (2011). Population coding of neural drive in human motor units during voluntary isometric contractions. Phd Thesis, Aalborg University.
Negro F, Holobar A & Farina D (2009). Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587, 5925–5938.
Williams ER & Baker SN (2009). Renshaw cell recurrent inhibition improves physiological tremor by reducing corticomuscular coupling at 10 Hz. J Neurosci 29, 6616–6624.
