Physiology News Magazine
Use it – don’t waste it: Facilitative urea transporters
Craig Smith explains why transport of urea across cell membranes plays many important roles in cell physiology –as well as some unexpected ones.
Features
Use it – don’t waste it: Facilitative urea transporters
Craig Smith explains why transport of urea across cell membranes plays many important roles in cell physiology –as well as some unexpected ones.
Features
Craig P Smith
School of Biological Science, University of Manchester
https://doi.org/10.36866/pn.45.20

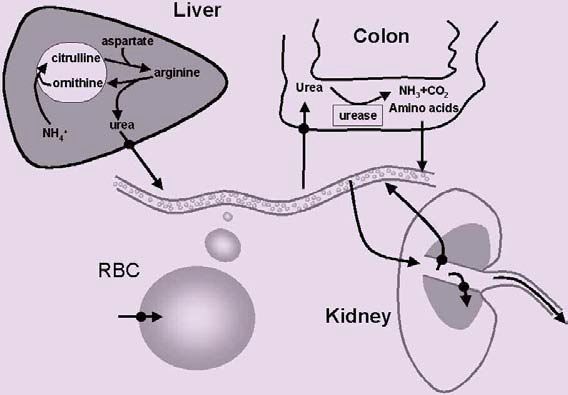
Urea is probably best known as the major end-product of amino acid breakdown in mammals. As a result of this, urea is often considered to be purely a waste product of metabolism. However, between 50-70% of the urea filtered by the kidney is actually reabsorbed, and urea plays important roles in mammalian water, and nitrogen, balance. Central to both these roles is regulation of the movement of urea across cell membranes.
Precisely how transmembrane urea movement is regulated has puzzled physiologists on and off since the pioneering work of Homer Smith in the 1930s. For many years it was widely believed that urea permeated membranes simply by lipid phase diffusion. However, this did not explain the high urea permeability of erythrocytes, or the fact that vasopressin stimulated a large increase in urea permeability in the kidney inner medulla. Indeed, the membranes of inner medullary cells, even in the unstimulated state, have a basal urea permeability much higher than can be attributed to lipid phase diffusion.
The isolation of a cDNA encoding a mammalian urea transporter protein in 1993 put an abrupt end to this debate and opened up a new field of solute transporter biology (You et al. 1993). This unique protein, termed UT-A2 (´Urea Transporter`), turns out to be a facilitative transporter, which acts, in effect, as a selective pore facilitating the diffusion of urea. Using homology screening techniques a family of urea transporters have now been characterised (see Smith & Rousselet 2001). All the urea transporters are the products of two closely related genes, UT-A and UT-B. Family members have now been detected in mammalian, elasmobranch, teleost and amphibian species.
Tissue distribution of urea transporters
In mammals the urea transporters show a wide tissue distribution. As predicted by functional studies, they are highly expressed in kidney, more precisely in the inner medullary collecting duct, and in parts of the thin descending limbs of the loop of Henle. Here the urea transporters are part of the so-called countercurrent multiplier, functioning to generate and maintain the high urea concentration in the medullary interstitium which powers water reabsorption. The capacity to harness urea as an osmolyte to drive water reabsorption across the kidney collecting duct must have been a significant evolutionary advance, allowing animals to leave the aquatic biosphere and exploit terrestrial habitats. Interestingly, elasmobranchs, which includes sharks, rays and skates, also use urea as an osmolyte, but not to concentrate urine. These animals have a plasma and tissue urea concentration of ~400mM, compared to 4-10mM in human plasma. This high urea concentration, in combination with NaCl, serves to raise the plasma and tissue osmolality to equal the 1000mOsm of the surrounding sea water. In this way elasmobranchs avoid the osmotic stress experienced by their bony teleost cousins, which maintain their internal milieu at 300mOsm.
Red Cells
Erythrocytes express UT-B transporters and some insight into the physiological role of this protein has been gleaned from studies involving humans with mutations in the UT-B gene. One genotype, known as Jknull, results in a total lack of UT-B function, yet Jknull individ- uals show no adverse symptoms. Their sole abnormality is that they cannot concentrate their urine to the same degree as people with functioning UT-B. This leads to the hypothesis that UT-B expressed in red cells, acting in concert with UT-B expressed in the kidney vasculature, contributes to the generation and maintenance of the high renal medullary interstitial urea concentration.
Liver
Urea is synthesised in the liver so it is not surprising that both UT-A and UT-B urea transporters are expressed in this tissue, where they are thought to provide a means of egress for newly synthesised urea into the circulation. Interestingly, an aquaporin water channel which is also permeable to urea, Aquaporin-9, is also expressed in liver. This protein may play a dual role as a diffusional pathway for both water and urea.
Urea, There, Everywhere
Expression of urea transporters in kidney, erythrocytes and liver was expected, given the role of these tissues in urea handling (Fig. 1). However, urea transporters have also been shown to be expressed in many tissues not associated with urea metabolism, including colon, testis (e.g. Fig. 2) and brain. In colon there is strong evidence in ruminants that intestinal breakdown of urea by resident microflora helps maintain nitrogen balance. We have found urea transporters expressed along the mouse gastrointestinal tract, raising the possibility that the same may be true in monogastric animals. If this turns out to be the case, then this mechanism may be important in people who consume a diet low in protein (which includes most of the third world), during illness when ingestion of food is disrupted, or during pregnancy when nitrogen demands are increased.
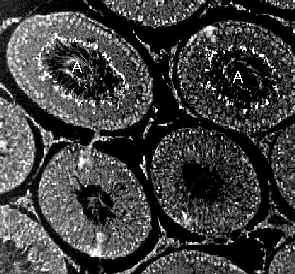
In testis the picture is less clear. We have found that urea transporter expression is coordinated to the spermatogenic cycle, implicating urea transporters in this process. However, there is little in the way of solid data to indicate what role urea transporters may play in this process. Urea is a by-product of the biogenesis of polyamines. Because the polyamines spermine and spermidine are important for DNA packaging, urea transporters may be required for removal of urea from the testis following the polyamine synthesis that accompanies DNA replication and packaging.
Another intriguing possibility is that urea transporters have other functions in addition to transporting urea. Ideas which remain to be fully tested include that they may transport other solutes, or may even have a dual role and function as something other than solute transporters. One hint of the latter is the finding that human UT-B complements the fission yeast (Schizosaccha-romyces pombe) rad1-1 cell cycle check-point mutation. Curiously, when over-expressed in a human cell line, UT-B induces apoptosis. It is difficult to explain these results strictly in terms of urea transport by UT-B, suggesting that UT-B may have a distinct function that is independent of urea transport. However, given that Jknull individuals are normal, apart from a mild urinary concentrating defect, this function is either non-essential or is made redundant in man by additional compensatory mechanisms.
To close, in the past decade the field of urea transporter biology has blossomed resulting in a profusion of molecular data. However, molecular data alone is insufficient to explain the role played by urea transporters in the diverse array of tissues where they are now known to be present. Future breakthroughs in this important field will only come from an integrated approach incorporating molecular and physiological techniques. Which can only be good news for us physiologists.
References
Smith CP & Rousselet G (2001). Facilitative urea transporters. J Membrane Biol. 183, 1-14.
You, G, Smith, CP, Kanai Y, Lee, W-S, Stelzner, M & Hediger, MA (1993) Expression cloning and characterisation of the vasopressin-regulated urea transporter. Nature, 365, 844-847.