
Physiology News Magazine
Using AI to develop better ovarian cancer treatment
Targeting residual cancer cells for future immunotherapy approaches
Features
Using AI to develop better ovarian cancer treatment
Targeting residual cancer cells for future immunotherapy approaches
Features

Professor Christopher Yau
Nuffield Department for Women’s & Reproductive Health, University of Oxford, Oxford, United Kingdom
Health Data Research UK, London, United Kingdom

Professor Ahmed Ashour Ahmed
Nuffield Department for Women’s & Reproductive Health, University of Oxford, Oxford, United Kingdom
NIHR Oxford Biomedical Research Centre, National Institute of Health Research, Oxford, United Kingdom
Artificial intelligence (AI) promises to provide a set of transformative technologies for healthcare. From automating the reading of medical images to optimising the flow of patients through hospitals, there are numerous applications of AI that are currently being explored internationally (Acosta et al., 2022).
In particular, the advent of molecular technologies that now enable us to routinely sequence our genomes and to measure the properties of single cells and molecules is providing new knowledge of the fundamental biochemical processes that govern normal and abnormal physiological behaviour. The data that arise from the use of these technologies are ripe for AI as the data quantity and richness defy manual human interpretation and necessitate the need for automated algorithmic solutions.
The convergence of molecular technologies and AI is being witnessed in their combined use in the development and implementation of advanced therapeutics for many diseases (Huang et al., 2022) and particularly for cancer (Ho, 2020). While cancer outcomes have improved for many disease types over the years, predicting response to treatment for individual patients remains challenging, and it is still unclear why some cancers are prone to relapse and treatment resistance, but others are not. In this article, we will examine how AI is being used to address these issues to advance therapeutic opportunities for one of the most challenging cancers to treat – ovarian cancer.
Ovarian cancer and prognosis
Over 300,000 cases of ovarian cancer were diagnosed globally in 2020, representing 1.7% of all cancer cases and making ovarian cancer the 18th most prevalent cancer. While breast cancer is the most prevalent (12.2%) (IACR, 2023), 85% of those diagnosed with breast cancer live for 5 years or more in the UK, whereas only 45% of ovarian cancer patients can expect the same life expectancy (NHS Digital, 2023). This makes ovarian cancers one of the cancers with the poorest patient outcomes and the most lethal of all the gynaecological cancers.
While ovarian cancers are predominantly sporadic, a subset of cases have a heritable genetic component. Individuals who inherit BRCA1/2 mutations, which are better known for causing breast cancers, are also at increased risk of ovarian cancers. Furthermore, Lynch syndrome, which significantly increases the risk of colorectal cancers, also increases the risk of ovarian cancer as well.
There are many types of ovarian cancer, with the high-grade serous subtype (HGSOC) the most common, representing 70% of cases and having the poorest prognosis (and the predominant focus of this article). Ovarian cancers are difficult to recognise as they are indicated by non-specific symptoms (such as abdominal bloating, irregular periods) and late diagnosis is common, which contributes to poor survival outcomes. When it is discovered, the cancer has often already spread outside the pelvis to the lining of the abdominal cavity (peritoneum). It can also migrate to the lymph nodes in the back of the abdomen.
Current treatments
Standard first-line treatment of ovarian cancer involves a combination of surgery to physically remove cancerous tissues and platinum-based chemotherapy. Platinum-based chemotherapies are cytotoxic agents that cause DNA damage by delivering platinum-based compounds into cells, which bind to DNA, causing adducts and cross-links in the DNA. These disrupt DNA replication and synthesis, triggering DNA repair mechanisms that are unable to resolve the damage and instead trigger apoptosis (cell death). Treatment relies on the principle that cancer cells divide more rapidly than non-cancer cells and will be disproportionately affected by the chemotherapy. Nonetheless, non-cancer cells will be impacted as well, and this results in patient side-effects such as neurotoxicity.
In the last decade, first-line ovarian cancer treatments have increasingly been combined with other approaches such as anti-angiogenic therapy or poly(ADP-ribose) polymerase (PARP) inhibitors.
Anti-angiogenic therapy targets angiogenesis – the mechanism for the formation of blood vessels. During tumour growth, cancer cells continuously secrete many related factors that promote angiogenesis, such that new vascular networks are continuously generated in the tumour tissues for the rapid proliferation of cancer cells. Anti-angiogenic therapies block key components of the angiogenesis pathway, such as vascular endothelial growth factor A (VEGF-A), to limit vessel formation and reduce tumour formation.
PARP inhibitors are targeted treatments for ovarian cancers that have mutations in the homologous recombinational repair (HRR) pathway, which is responsible for resolving double-strand DNA breaks. PARP inhibitors block the function of the PARP enzyme, which is involved in the repair of single-strand DNA breaks. During DNA replication, these unrepaired single-strand DNA breaks are converted into double-strand breaks that cancer cells with deficient HRR function cannot repair, which then triggers cell death.
Despite the availability of these treatments for patients diagnosed with late-stage ovarian cancers, over 70% will have a recurrence with a median time to relapse of 18-24 months. Relapsed disease is generally incurable, and the objective of further therapy is to control the condition and manage symptoms, prolong the need for further treatment, and maintain quality of life for the patient.
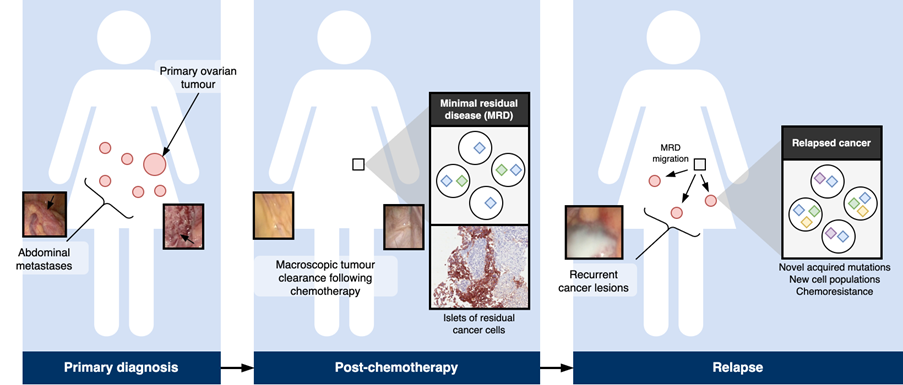
Residual cells, the seeds to cancer cell repopulation
High-resolution studies of post-operative tissues reveal that microscopic deposits of ovarian cancer cells (minimal residual disease, MRD) can remain after surgery and chemotherapy (Hellner et al., 2016). These residual cells are the seeds by which the cancer cell population can repopulate and, having already survived a course of treatment, they are likely to be resistant to further applications of the same treatments (Fig.1).
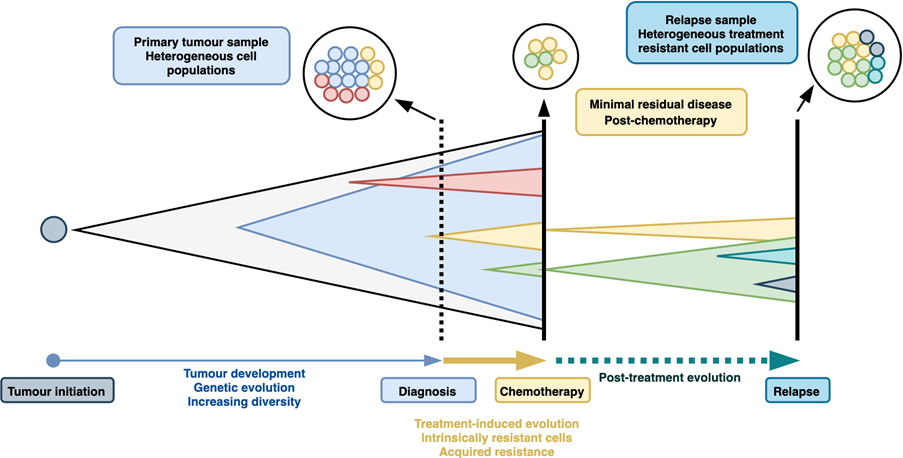
Many biological mechanisms have been attributed to treatment resistance in ovarian cancer. Underpinning these is the genetic diversity of ovarian cancers (Fig.2). Whole-genome sequencing has revealed that HGSOCs contain anywhere between 5,000 and 50,000 single nucleotide mutations as well as a complex landscape of chromosomal rearrangements and DNA copy number changes (Cancer Genome Atlas Research Network, 2011). The characterisation of these mutational processes has led to the identification of mutational signatures (Macintyre et al., 2018) and pathways leading to different evolutionary states and trajectories (Lahtinen et al., 2023). Mutations lead to alterations in normal cellular programming that promote the growth of the cancer and may confer resilience to treatments. For example, cancers that acquire reversion mutations that restore HRR function can become resistant to PARP inhibitors. The mutational processes driving ovarian cancers are continual; new mutations are acquired over time and as the cancer grows. This presents a challenging dynamic target for treatments and explains why standard treatments can eventually be overcome by cancer cells.
Non-genetic mechanisms could also contribute to treatment resistance. We proposed a model whereby the phenotypic behaviour of HGSOC cells is a mixture of five signatures, which we identified through single-cell sequencing of the cell-of-origin (the fallopian tube epithelium) of ovarian cancers (Hu et al., 2020). We have also observed that MRD cells harbour an adipocyte-like gene expression signature (Artibani et al., 2021). Using a cell culture model, MRD-mimic cells were found to be dependent on fatty acid oxidation for survival and displayed resistance to cytotoxic agents. Treatment-induced cell plasticity can therefore push ovarian cancers towards phenotypic states that confer greater resistance to therapies.
Interactions between ovarian cancer cells and adjacent stromal and immune cells within the tumour microenvironment (TME) also provide a further source of resistance pathways. For example, ovarian cancers have been associated with increased levels of cancer-associated fibroblasts (CAFs) that can obstruct the transportation of chemotherapeutic agents and impair chemotherapeutic efficacy by creating physical barriers and microvascular compression, while ovarian cancers classified as having a high level of epithelial-to-mesenchymal transition expression markers have been found to have a particularly poor prognosis and were associated with high frequency of immunosuppressive macrophages (Hu et al., 2021).
Creating new DNA sequencing tools
To examine the exact properties of treatment-resistant cancer cells, we must collect them immediately following treatment. However, retrieving and analysing post-treatment ovarian cancer specimens is difficult. Sample retrieval requires invasive procedures and, due to the microscopic nature of residual disease, only a small amount of biological material may be available. This can present challenges for subsequent analysis. If we wait until the disease relapses, more diseased tissue would be available but, as the cancer evolves over time, the properties of the cancer may have changed in the time between primary treatment and relapse.
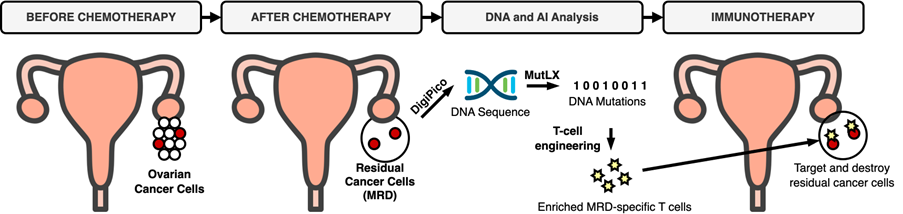
Small islets of residual disease may contain fewer than 100 cells but standard whole-genome sequencing typically requires large quantities of DNA obtained from tens of thousands of cells. We developed DigiPico (Carrami et al., 2020), a sequencing protocol specifically designed to enable us to sequence the picograms of DNA available from residual disease samples. In addition to the experimental techniques, it was also necessary to develop an AI algorithm, called MutLX, to process the sequencing data to reliably differentiate true mutations from artefacts. MutLX adapts its behaviour, adjusting to the noise and sequencing characteristics of each sequenced sample. The result is that we are now able to examine the genetic properties of microscopic residual disease cell populations and investigate resistance mechanisms in ovarian cancer.
The future of ovarian cancer treatment
AI technologies will change the way ovarian cancer is diagnosed and treated within the next 10-15 years. The most immediate impacts will likely emerge first in diagnostic tools. As different types of clinical, molecular, and imaging technologies become available, data arising from these will need to be integrated via AI to support clinicians with treatment planning and selection. Later, what is perhaps most exciting is that AI might become integrated into the production of patient-specific treatments as well.
For example, adoptive T-cell therapies attempt to enhance a patient’s own immune system’s ability to attack and eradicate their tumour with T-cells (Morotti et al., 2021). These therapies involve removing a patient’s or donor’s T-cells, growing and/or modifying them in a laboratory, and reinfusing them back into the patient (Fig.3). One challenge with these therapies is the phenomenon known as “T-cell exhaustion”.
Mutations carried by cancer cells appear foreign to our immune system, which reacts by initiating a T-cell response to eliminate the cancer. However, if the immune system is persistently unable to clear the cancer, T-cell exhaustion can occur, in which the immune system switches from attacking the cancer to co-existing with it and T-cells become underactive. Boosting the cancer-specific T-cell response through adoptive T-cell therapy is an obvious approach to addressing this issue. Using DigiPico and MutLX, we can now sequence and identify mutations specific to residual cancer cells, and therefore identify the corresponding cancer-specific T-cell subpopulation. By expanding and enriching this subpopulation in the laboratory and then reimplanting these T-cells into the patient, the hope is that the residual cancer could be fully eradicated, preventing relapse. Importantly, by intervening at the MRD stage, the disease can be tackled before further cancer evolution takes place, which might alter the properties of the disease.
Modern vaccine technologies have also been developed as cancer therapeutics (Lin et al., 2022). Messenger RNA-based cancer vaccines deliver short RNA fragments into the body, which instruct cells that take up the vaccine to produce tumour-associated proteins that stimulate an immune response against the patient’s cancer. AI algorithms are used to identify the target signatures but can also be used to design and optimise the design of the mRNA vaccine itself to maximise stability and specificity of the treatment.
These examples suggest the tantalising possibility of combining AI to aid in the more accurate diagnosis and prognosis of a patient’s cancer with AI that helps to create bespoke therapeutics to address the condition in the future. However, this use of AI also presents new regulatory challenges. For instance, if every ovarian cancer patient receives therapy that is uniquely tailored to their cancer at the molecular level, how do we evaluate the effectiveness of the therapy if there is only one person who receives that exact therapeutic? These and other issues such as the potential for bias to exist in AI systems or that AI may be overconfident about its own predictions necessitate the need for detailed thinking and planning in determining the overall benefit of AI-enabled therapeutic pathways. Nonetheless, after decades in which the long-term outcomes of ovarian cancer patients have not significantly changed, the possibility of new approaches to treating this condition is an enormously exciting opportunity.
References
Acosta JN et al. (2022). Multimodal biomedical AI. Nature Medicine 28(9), pp.1773-1784.
Artibani M et al. (2021). Adipocyte-like signature in ovarian cancer minimal residual disease identifies metabolic vulnerabilities of tumor-initiating cells. JCI Insight 6(11).
Cancer Genome Atlas Research Network (2011). Integrated genomic analyses of ovarian carcinoma. Nature 474(7353), p.609.
Carrami EM et al. (2020). A highly accurate platform for clone-specific mutation discovery enables the study of active mutational processes. Elife 9, p.e55207.
Hellner K et al. (2016). Premalignant SOX2 overexpression in the fallopian tubes of ovarian cancer patients: Discovery and validation studies. EBioMedicine 10, pp.137-149.
Ho D (2020). Artificial intelligence in cancer therapy. Science 367(6481), pp.982-983.
Huang K et al. (2022). Artificial intelligence foundation for therapeutic science. Nature Chemical Biology 18(10), pp.1033-1036.
Hu Z et al. (2020). The repertoire of serous ovarian cancer non-genetic heterogeneity revealed by single-cell sequencing of normal fallopian tube epithelial cells. Cancer Cell 37(2), pp.226-242.
Hu Z et al. (2021). The Oxford Classic links epithelial-to-mesenchymal transition to immunosuppression in poor prognosis ovarian cancers. Clinical Cancer Research 27(5), pp.1570-1579.
IACR (2023). Global Cancer Observatory. https://gco.iarc.fr.
Lahtinen A et al. (2023). Evolutionary states and trajectories characterized by distinct pathways stratify patients with ovarian high grade serous carcinoma. Cancer Cell 41(6), pp.1103-1117.
Lin MJ et al. (2022). Cancer vaccines: the next immunotherapy frontier. Nature Cancer 3(8), pp.911-926.
Macintyre G et al. (2018). Copy number signatures and mutational processes in ovarian carcinoma. Nature Genetics 50(9), pp.1262-1270.
Morotti M et al. (2021). Promises and challenges of adoptive T-cell therapies for solid tumours. British Journal of Cancer 124(11), pp.1759-1776.
NHS Digital (2023). Cancer Survival in England. URL: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-survival-in-england/cancers-diagnosed-2016-to-2020-followed-up-to-2021/survival-by-cancer-group.
FDA (2019). https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics.
