Physiology News Magazine
What does the future hold for research into Epithelia & Membrane Transport?
What is the most important property of an epithelium?
Features
What does the future hold for research into Epithelia & Membrane Transport?
What is the most important property of an epithelium?
Features
James Garnett
Boehringer Ingelheim, Germany, and Newcastle University, UK
Morag Mansley
The University of Edinburgh, UK
Co-convenors of the Epithelia and Membrane Transport theme
https://doi.org/10.36866/pn.107.36
As a PhD student I was asked the question: ‘what is the most important property of an epithelium?’ I tried my best to recite some typical textbook phrases like how it acts as a selective barrier to the external environment and its transcellular transport capabilities. On all counts I was wrong – the answer my superior was looking for was that ‘the cells are polarised’.


Some 10 years on, I cannot help but wonder what answer I would receive to this same question if I were to ask a PhD student in an epithelia research lab today. Of course, some may indeed still answer ‘epithelial polarity’, but possibly for less obvious reasons than the distinct plasma membrane domains allowing directional transport of molecules across the epithelium. In cancer research, loss of epithelial polarity marks the beginning of epithelia-to-mesenchymal transition in which cells acquire migratory and invasive capabilities, promoting metastasis in epithelium-derived carcinoma. A student in an epithelial barrier lab may also give this answer but in the context of epithelial wound healing, where epithelial polarity defines cellular differentiation vs proliferation potential. And in an infectious disease lab the same answer could be used to point to how certain bacterial pathogens are capable of reversing epithelial polarity as a mechanism of penetrating the epithelial barrier.
My guess would be that many would give much more diverse answers to this question, and I can only imagine what might be considered the most fundamental properties of an epithelium in another 10 years’ time. In the context of the epithelium as a protective barrier against pathogens, new research into barrier properties and host–microbiome crosstalk could provide innovative alternative approaches to overcome inflammatory diseases affecting the intestinal and respiratory mucosa. Indeed new insights into the innate immune signalling or potential phagocytic capacity of epithelial cells could even see epithelia classified in physiology textbooks of the future as a type of immune cell. Beyond the well-studied tight and adherens junctions, new innovations in microscopy are expanding our understanding of epithelia cell–cell communication such as how deep nuclear invaginations form tunnels traversing the nucleus and encasing the cytoskeleton, providing a physical/mechanical link from the nucleus to desmosomes at the cell membrane.
And all this before one even begins to consider the possibilities of future research into epithelial membrane transport, an exciting field of research that has provided new therapies for many diseases and most notably in recent years has provided the closest thing to a cure for a small subset of Cystic Fibrosis patients through insights into the regulation of the CFTR ion channel. New insights into novel aspects of the regulation of membrane transporters and channels, such as the study of extracellular vesicles, may represent the next major breakthrough in the identification and treatment of the numerous disease states in which ion and solute transport become dysregulated.
Extracellular vesicles: novel regulators of epithelial transport?
One of the defining features of a polarised epithelium is its ability to transport a variety of different ions and solutes, as well as water. This occurs via transporters and ion channels differentially expressed within distinct apical and basolateral domains of the plasma membrane.
Membrane transport across epithelial tissues is subject to regulation by a wide variety of hormones and bioactive factors, which can either upregulate or downregulate transport processes. For instance, in response to increased plasma osmolality, release of the peptide hormone arginine vasopressin
(AVP) promotes water reabsorption by inserting water channels within the renal collecting duct. These regulatory processes typically involve receptor-mediated signal transduction with resulting alterations to number and/or activity of transporters.
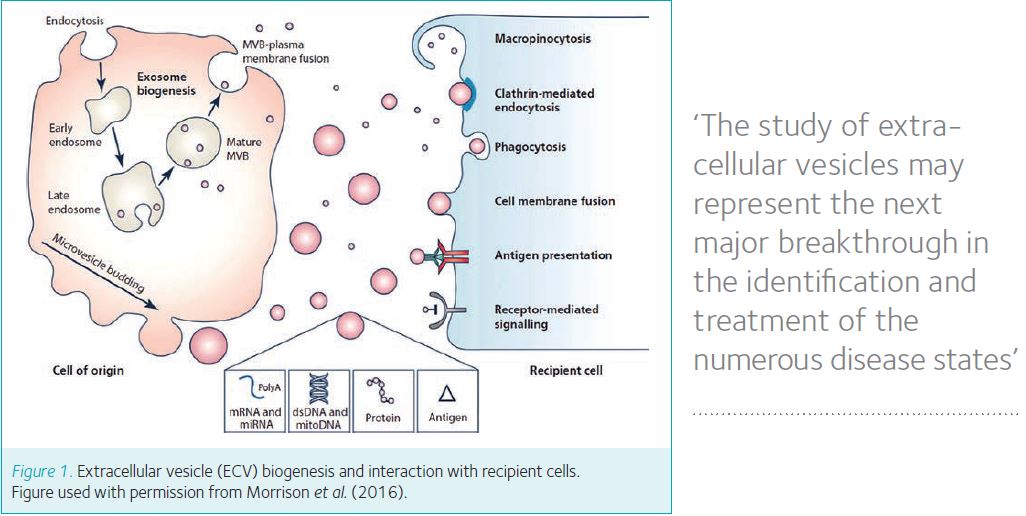
A novel route of cellular signalling has received a great deal of interest recently, namely the release of extracellular vesicles (ECVs) from cells. These nano-sized vesicles are released from nearly all cell types including neurons and immune cells, as well as epithelial cells. ECVs are typically classified by their size, origin and identifying markers, and multiple methods are now well established to isolate and identify ECVs (reviewed recently in J Physiol; Morrison et al., 2016). ECVs have been proposed to carry cargo including proteins, lipids and RNA which upon release from the donor cell can be transferred and incorporated into a recipient cell (Fig. 1).
The identification of ECVs in human urine samples demonstrated that epithelial cells spanning the length of the nephron are capable of releasing these vesicles (Pisitkun et al., 2004). Analysis by mass spectrometry revealed these ECVs contained a variety of proteins including tubular transporters as well as proteins involved in membrane trafficking. Interestingly, analysis of ECVs in urine collected from patients with Bartter Syndrome Type 1 showed a complete absence of the Na-K-Cl co-transporter 2 (NKCC2), revealing a potential use of ECVs as biomarkers of disease (Gonzales et al., 2004). Indeed, biomarkers in urinary ECVs have been proposed across the entire spectrum of renal disease (Morrison et al., 2016). The value of these potential biomarkers remains to be determined, and only large-scale population studies will reveal if these will be of clinical use in the future.
In terms of intercellular signalling, studies in renal epithelial cells have demonstrated that cells not only release ECVs, they can also take up ECVs. Importantly the contents of these vesicles can be transferred, as shown by the incorporation of ECVs with functional AQP2 water channels, collected from AVP-stimulated donor cells, into unstimulated recipient cells (Street et al., 2016). In addition to the transfer of transporter proteins, there is now evidence that ECVs contain micro RNAs (miRNAs) which can alter epithelial transport (Gracia et al., 2017; Oosthuyzen et al., 2017). Interestingly, the targets of identified miRNAs are not only the transporters themselves, but intracellular kinases known to regulate them.
The physiological relevance of this form of cellular signalling remains to be determined. In what context does ECV signalling become important? Moreover, what additional control does ECV signalling exert alongside the more classical hormone/receptor-mediated regulation of epithelial transport? The potential for cellular signalling along the length of the nephron via ECVs in the ultrafiltrate certainly seems plausible and may indeed be of great relevance.
References
Gonzales PA et al. (2009). Large-scale proteomics and phosphoproteomics of urinary exosomes. Journal of the American Society of Nephrology
20, 363–379. [DOI: 10.1681/asn.2008040406]
Gracia T et al. (2017). Urinary exosomes contain microRNAs capable of paracrine modulation of tubular transporters in kidney. Sci Rep 7, 40601.
[DOI: 10.1038/srep40601]
Morrison EE, Bailey MA, Dear JW (2016). Renal extracellular vesicles: from physiology to clinical application. J Physiol 594, 5735–5748. [DOI: 10.1113/jp272182]
Oosthuyzen W et al. (2016) Vasopressin regulates extracellular vesicle uptake by kidney collecting duct cells. Clin J Am Soc Nephrol 27, 3345–3355.
[DOI: 10.1681/asn.2015050568]
Pisitkun T, Shen RF, Knepper MA (2004). Identification and proteomic profiling of exosomes in human urine. PNAS 101, 13368–13373. [DOI: 10.1073/pnas.0403453101]
Street JM et al. (2011). Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J Physiol 589, 6119–6127.