
Physiology News Magazine
What happens to the central respiratory rhythm during breath-holding?
Michael Parkes considers some of the implications of the continuation of the central respiratory rhythm during breath-holding
Features
What happens to the central respiratory rhythm during breath-holding?
Michael Parkes considers some of the implications of the continuation of the central respiratory rhythm during breath-holding
Features
Michael J Parkes
School of Sport & Exercise Science, University of Birmingham, UK
https://doi.org/10.36866/pn.68.16

Humans have virtually no voluntary control of their heart beating, but do have considerable voluntary control of breathing. For example, they can easily synchronise breathing to a metronome over the range of 5–30 beats per minute. There has always been a tacit presumption that humans stop their respiratory rhythm during breath-holding. Recent evidence shows, however, that this is not the case.
It is not yet possible to measure the central respiratory rhythm directly in humans. Recordings cannot yet be made from brainstem neurones with respiratory-related discharge, nor from the phrenic nerve. (Conversely, animal training has so far failed to elicit breath-holding experiments longer than about 5 seconds.) In humans, the activity of the central respiratory rhythm is normally inferred from respiratory motor output such as inspiratory pressure, airflow or chest movement. Obviously, such respiratory motor output stops during breath-holding, but this doesn’t necessarily establish that the central respiratory rhythm has stopped.

In a classic study in 1963, Emilio Agostoni had three subjects swallow a catheter, enabling measurement of oesophageal pressure and electromyogram activity (emg). He showed (Fig. 1A) that rhythmic emg bursts appeared towards the end of the breath-hold. Since each burst was accompanied by a wave of negative pressure, the emg activity was believed to be recorded across the oesophagus from the diaphragm. Subsequent studies by William Whitelaw showed that the amplitude and frequency of these negative pressure waves increased towards the end of the breath-hold. Since PCO2 rises linearly throughout breath-holding, these waves possess the CO2 sensitivity characteristic of the central respiratory rhythm. These studies show that the central respiratory rhythm reappears before the breakpoint of breath-holding. They do not, however, establish whether the central respiratory rhythm suddenly reappears, or whether it had not stopped but was simply too difficult to detect at the start of breathing.
We have developed another approach to reveal the presence of the central respiratory rhythm, by using respiratory sinus arrhythmia. The factors contributing to sinus arrhythmia are complex. Animal studies show that these include effects of the central respiratory rhythm and of pulmonary inflation and its mechanical sequelae (including complex interactions with the baroreflex and atrial stretch). No study has established the precise contribution each makes to total respiratory sinus arrhythmia.
We therefore established first that the central respiratory rhythm does make a substantial contribution to sinus arrhythmia in unanaesthetised humans (Cooper et al. 2004). We did this by devising a regime of mechanical hyperventilation, where the central respiratory rhythm remained demonstrable in normocapnia from respiratory motor output (diaphragm emg activity recorded from the chest surface and from the shape of the inflation pressure waveform). We maintained normocapnia (PetCO2 of 41 mmHg) during hyperventilation by addition of CO2 to the inspirate. In humans, hypocapnia augments the potency of the pulmonary stretch reflex but attenuates the central respiratory rhythm, so it is an ideal condition to reveal the predominant contribution to respiratory sinus arrhythmia. We induced hypocapnia (24 mmHg) by a surreptitious removal of CO2 from the inspirate, which was imper-ceptible to subjects. We showed that during hypocapnia the rhythmic changes in pulmonary inflation were unchanged but the respiratory motor output disappeared i.e., the central respiratory rhythm was greatly attenuated. The fact that there was also a large reduction in sinus arrhythmia establishes that the central respiratory rhythm makes the predominant contribution to respiratory sinus arrhythmia in humans.
In some ways, breath-holding is an ideal way to reveal whether the central respiratory rhythm continues to cause respiratory sinus arrhythmia, because rhythmic pulmonary inflation and its mechanical sequelae are absent. There has been much debate about whether respiratory sinus arrhythmia does continue during breath-holding. The Handbook of Physiology seems to suggests that it does not, yet it is clearly evident in some early studies (e.g. Valentinuzzi & Geddes, 1974). Much of the debate arises because of the difficulties in using relatively short breath-holds (20–45 s). If some subjects happen to breathe naturally at relatively low frequencies (e.g. < 6 breaths per minute), there would be so few respiratory cycles that respiratory sinus arrhythmia may well be difficult to identify.
We avoided these difficulties (Cooper et al. 2003) by prolonging breath-hold duration to a mean of 4 minutes with preoxygenation. (Preoxygenation has the additional advantage of preventing the chemoreflex stimulation of heart rate that normally occurs during breath-holding.) Fig. 2(e) shows that sinus arrhythmia does continue from the start of breath-holding. This sinus arrhythmia is also CO2 sensitive. The spectral power density measurements indicate that sinus arrhythmia moves to higher frequencies as pCO2 increases throughout the breath-hold and that there is less sinus arrhythmia in breath-holds from hypocapnia.
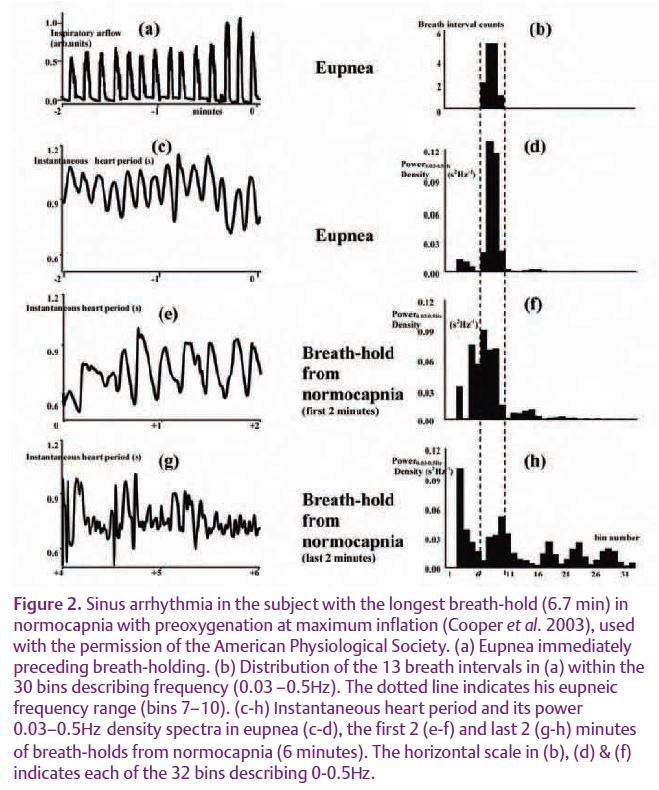
In 2006 I was able to perform a similar analysis on some of Emilio Agostoni’s 1963 data when he kindly lent me some of his original records. From enlargements I was able to measure sinus arrhythmia from the ECG artefacts and its relationship to the oesophageal emg and pressure waves (Parkes, 2006). Fig. 1B shows, in this 1963 data too, that sinus arrhythmia persists from the start of breath-holding. Furthermore, Fig. 1C shows that it coincides with the rhythmic emg and negative pressure waves when they appear. In the absence of direct measures of the central respiratory rhythm in humans, the persistence of CO2-sensitive sinus arrhythmia throughout breath-holding provides the best evidence that the central respiratory rhythm does continue from the start of breath-holding.
This has a number of important implications for our understanding of basic human physiology. First, humans clearly have less voluntary control of their central respiratory rhythm than was previously supposed. It would be far too dangerous to be able to stop the heart beating voluntarily. It now appears that humans also lack this level of control over their other vital rhythm. This, in turn, has interesting implications for our understanding of ‘apnea’ and ‘central apnea’ and their clinical diagnosis and treatment.
Second, many previous studies (particularly in cardiovascular control), have used breath-holding to study mechanisms in the presumed absence of the central respiratory rhythm. This presumption is no longer valid. All that is absent is rhythmic pulmonary inflation. Similarly, it is no longer correct to presume either that end-expiratory breath-holds stop the central respiratory rhythm in its expiratory phase, or that end-inspiratory breath-holds stop it in the inspiratory phase. Indeed, for breath-hold research, the lung volume itself may be the only important feature, not the means by which it is achieved.
Finally, the mechanism of breath-holding is not voluntary cessation of the central respiratory rhythm but is voluntary suppression of the expression of this rhythm. The latest techniques of brain imaging might be useful in identifying the location(s) of this suppression.
References
Agostoni E (1963). Diaphragm activity during breath holding: factors related to its onset. J Appl Physiol 18, 30–36.
Cooper HE, Clutton-Brock TH, & Parkes MJ (2004). The contribution of the respiratory rhythm to sinus arrhythmia in normal unanesthetized subjects during mechanical hyperventilation with positive pressure. Am J Physioly 286, H402–H411.
Cooper HE, Parkes MJ, & Clutton-Brock TH (2003). CO2-dependent components of sinus arrhythmia from the start of breath-holding in Man. Am J Physiol 285, H841–H848.
Parkes MJ (2006). Breath-holding and its breakpoint. Exp Physiol 91, 1–15.
Valentinuzzi ME & Geddes LA (1974). The central component of the respiratory heart-rate response. Cardiovascular Research Center Bulletin 12, 87–103.
Whitelaw WA, McBride B, Amar J, & Corbet K (1981). Respiratory neuromuscular output during breath holding. J Appl Physiol 50, 435–443.
Whitelaw WA, McBride B, & Ford GT (1987). Effect of lung volume on breath holding. J Appl Physiol 2, 1962–1969.
