Physiology News Magazine
What makes some axons more excitable than others?
Have you ever wondered why some motor axons may be activated before all others in a muscle as an electrical stimulus is increased? It could just be that the low threshold represents a quirk of anatomy and current flow, but it could also be that the excitability of the axon is greater than that of other axons innervating the muscle. The latter seems to be the case and a key factor seems to be the hyperpolarisation-activated current (Ih)
Features
What makes some axons more excitable than others?
Have you ever wondered why some motor axons may be activated before all others in a muscle as an electrical stimulus is increased? It could just be that the low threshold represents a quirk of anatomy and current flow, but it could also be that the excitability of the axon is greater than that of other axons innervating the muscle. The latter seems to be the case and a key factor seems to be the hyperpolarisation-activated current (Ih)
Features
Louise Trevillion (1), James Howells (1), Susan Tomlinson (1), Hugh Bostock (2) and David Burke (1)
1: Institute of Clinical Neurosciences, Royal Prince Alfred Hospital and The University of Sydney, Sydney, Australia
2: Sobell Department of Neurophysiology, Institute of Neurology, Queen Square, London, UK
https://doi.org/10.36866/pn.82.32

The excitability properties of human axons can be studied non-invasively by tracking the changes in the strength of the electrical stimulus required to generate an action potential. This technique is known as threshold tracking.
We teach that the largest nerve fibres not only conduct faster but are also easier to excite electrically than smaller axons. Small axons arising from small motoneurones are recruited first by voluntary effort or by reflex inputs (according to Henneman’s size principle; Henneman et al. 1965). On the other hand, sensory axons in human peripheral nerve are excited electrically at lower threshold than motor axons due, in part, to a difference in sodium channels.
Now a study using the threshold tracking of human motor axons has shown that the first motor axons to be excited electrically are those with greater activity of the hyperpolarization-activated current, Ih. Ih is a depolarizing current that plays a pacemaker role in the heart and CNS and is due to hyperpolarization-activated cyclic nucleotide-gated (HCN) channels.
This previously unsuspected role of HCN channels in controlling the resting excitability of axons may be linked to their role in the hyperexcitability of the nociceptive afferents responsible for neuropathic pain.
Sensory and motor axons
Differences in membrane conductances between sensory and motor axons have been demonstrated in human peripheral nerves. There is a significant difference in the accommodation to hyperpolarization between sensory and motor axons (shown as changes in excitability in Fig. 1).
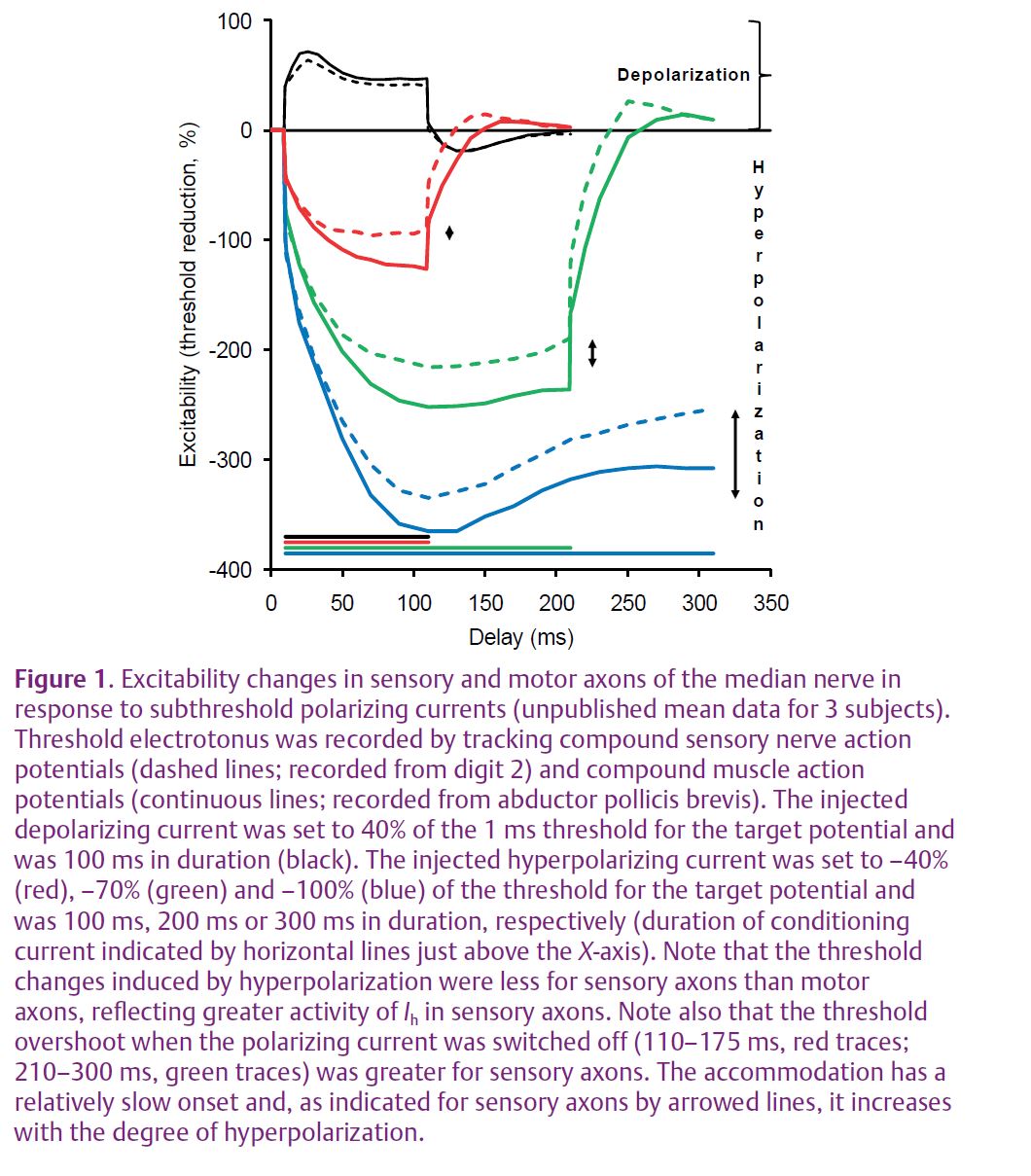
Sensory axons appear to have a greater activity of Ih (Fig. 1; Bostock et al. 1994). It is believed that the role of the current is to limit activity-dependent hyperpolarization following the conduction of trains of impulses. Sensory axons also have a greater persistent Na+ conductance (Bostock & Rothwell, 1997), reflected in a longer strength–duration time constant (Panizza et al. 1992). This Na+ conductance is active at threshold and contributes to resting membrane potential. These differences have been suggested to render sensory axons more susceptible to ectopic activity than motor axons, while making them less susceptible to conduction block.
In addition to differences between sensory and motor axons, there are differences within an axon type. It is well documented that motor units of a muscle differ in their motoneurone properties and the mechanical and histochemical properties of the innervated muscle fibres. Motor units are remarkably consistent in their recruitment thresholds, whether they be in voluntary or reflex contractions on the one hand, or in response to electrical stimululation on the other. All things being equal, voluntary effort should recruit motoneurones with smaller, more slowly conducting axons first (Henneman et al. 1965), while electrical stimulation should activate larger axons first. There are, however, a number of factors which need to be taken into account. In studies in human subjects, stimulation is percutaneous, at some distance from the closest nodes of Ranvier. Do proximity to the stimulus and anatomical factors determine which axons are first recruited from any one site rather than variations in intrinsic excitability due to differences in their biophysical properties? If low threshold is a reflection of increased excitability, what are the determinants?
Nerve excitability and threshold tracking
The excitability properties of human axons can be studied non-invasively by tracking the changes in the strength of an externally applied electrical stimulus required to generate an action potential. (Typically adhesive ECG electrodes are used with the cathode positioned over the nerve and the anode placed remotely on electrically less excitable tissue nearby.) The stimulus is submaximal, and is adjusted by computer to activate a preset submaximal nerve volley (usually 40–50% of maximum). The nerve volley generates a compound muscle action potential (CMAP), recorded using surface electrodes placed over the belly of the innervated muscle, and a compound sensory nerve action potential (CSNAP or, more simply, SNAP) recorded with ring electrodes placed around the innervated digit. If axons become depolarized (for example, during ischaemia induced by inflation of a pressure cuff), they are more excitable and a smaller current will be required to produce the target potential. If axons become hyperpolarized (for example, after conducting trains of impulses during a sustained muscle contraction), they are less excitable and more current is required. This technique of threshold tracking provides measures of excitability which are determined by the electrical properties of the axonal membrane at the point of stimulation. The procedure differs from nerve conduction studies in which a supramaximal stimulus is used to ensure that all axons within the nerve are excited.
The field of nerve excitability owes a debt to Joseph Bergmans who, in 1970, documented the excitability properties of single human motor axons. He found that single motor axons could be selectively activated over a large range of stimulation strengths and that the same motor units could be activated by threshold stimuli on repeated occasions when the stimulating electrodes were placed in the same location. He defined threshold as the lowest stimulation strength that would elicit three to five consecutive motor unit potentials (a motor unit potential is the potential generated by the muscle fibres innervated by a single motor axon) and then painstakingly adjusted stimulus voltage by hand to track changes in the threshold of the axon. He made meticulous and methodical recordings from single motor units in order to study recovery from single and repetitive activation and the influence of ischaemia, electrical polarization and temperature on these processes. From these non-invasive, in vivo studies, Bergmans was able to describe the mechanisms responsible for the different phases of post-tetanic hyperpolarization.
The development of an automated technique for tracking the threshold of a compound sensory or muscle action potential (Bostock et al. 1998) gave researchers a remarkable tool that allowed them to rapidly assess axonal excitability. In a matter of minutes, the stimulus–response relationship, strength–duration properties, accommodation to subthreshold changes in membrane potential and recovery after activation can be measured, providing information about both nodal and internodal conductances. The measurements are made non-invasively and in real time, allowing the time course of interventions such as drug administration and renal dialysis to be followed.
Many studies have been conducted using this tool and there is now a considerable body of literature describing findings in normal nerves and in numerous pathophysiological conditions ranging from nerve disorders, such as channelopathies and demyelinating conditions, to metabolic disorders, such as renal failure and diabetes. Further, differences between the excitability properties of axons in the upper and lower limbs and also between the proximal and distal segments within the same nerves have been found. The technique has also been successfully applied to the study of animal nerves (and single axons), giving rise to a body of literature on the changes in axonal excitability with maturation.
Excitability properties of single motor axons
Automated threshold tracking has recently been applied to investigate the excitability of selectively activated single motor units (a motor unit being the motoneurone, its motor axon and the muscle fibres it innervates) to determine whether the reason that they have a low threshold to electrical recruitment is due to intrinsic properties (Trevillion et al. 2010). Threshold tracking enabled comparisons to be made between the excitability properties of the single motor axons with those of higher threshold motor axons recruited in compound potentials recorded in the same experimental session.
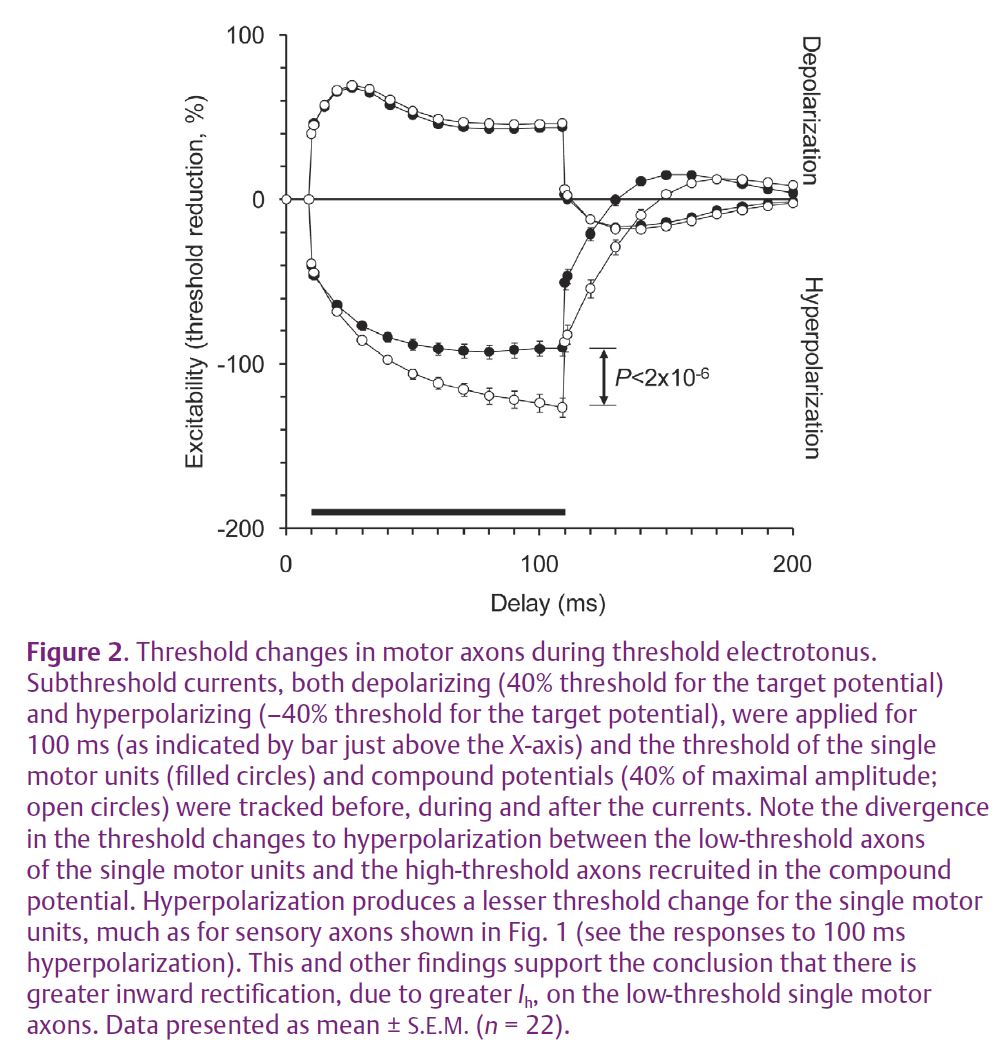
Based on earlier published findings on the ionic determinants of threshold (Bostock & Rothwell, 1997), it was anticipated that a difference in the persistent Na+ conductance between the low- and high-threshold axons would be revealed. However, this proved not to be the case. The key point of differentiation between the axons was found to be in the accommodation to hyperpolarizing currents (Fig. 2). Low-threshold axons were found to have greater accommodation to hyperpolarizing changes in membrane potential, presumably due to a greater hyperpolarization-activated inwardly rectifying conductance, Ih. This conductance has been shown to have a pacemaker role in spontaneously firing neurones and the sinoatrial node. HCN channels mediate the inwardly rectifying conductance and are known to exist in four isoforms. All have been found in the mammalian nervous system, although channel expression in the human peripheral nervous system is yet to be characterised. The findings for single motor axons suggest that HCN channels provide a depolarizing current at rest and therefore play a role in setting membrane potential.
Physiological implications
The above findings suggest that the motor axons that are less readily recruited by voluntary activation may have a greater activity of Ih and may, therefore, be more secure against the development of conduction block in disease processes. The flipside is that the increase in excitability makes these axons more prone to ectopic activity, such as fasciculation, and perhaps to motoneurone loss in amyotrophic lateral sclerosis. A corollary of this is that axons from smaller motoneurones, recruited more readily into voluntary contractions, may be more likely to develop conduction block when hyperpolarized during activity. This has implications for conditions such as multiple sclerosis, chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy, where these conduction abnormalities occur.
The stimulus–response curve for motor axons is relatively steep, reflecting minimal variation in threshold. However, the above findings demonstrate a significant variation in the intrinsic properties of different motor axons. This may have implications for some motor unit number estimation (MUNE) techniques that are based on the ‘mean’ motor unit measured using surface electromyography.
Spontaneous discharges from injured peripheral nerves are believed to be responsible for the induction and maintenance of neuropathic pain syndromes. Upregulation of Ih in injured nerves is believed to be a factor in the spontaneous action potentials (Chaplan et al. 2003), making HCN channels the targets for pharmacological treatments for neuropathic pain. Just as Ih expression varies between motor axons, resulting in threshold variation, it may be that Ih expression varies considerably between sensory axons rendering some more prone to generating the spontaneous activity seen in neuropathic pain syndromes.
Computer-controlled threshold tracking provides a reliable method for assessing Ih expression in human motor and sensory axons, non-invasively by tracking compound action potentials (Tomlinson et al. 2010). In fact, threshold tracking could be used to assess the efficacy of HCN channel blockers in animal models of neuropathic pain and to monitor the effects of these agents on axonal function.
Hugh Bostock and colleagues run occasional 3-day, hands-on nerve excitability workshops, which provide a detailed introduction to the theory and practice of nerve excitability testing. Those interested are invited to email h.bostock@ion. ucl.ac.uk for further information.
References
Bergmans J (1970). The Physiology of Single Human Nerve Fibres. Vander, University of Louvain, Belgium.
Bostock H, Burke D & Hales JP (1994). Differences in behaviour of sensory and motor axons following release of ischaemia. Brain 117, 225–234.
Bostock H, Cikurel K & Burke D (1998). Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21, 137–158.
Bostock H & Rothwell JC (1997). Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol 498, 277–294.
Chaplan SR, Guo H-Q, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM & Dubin AE (2003). Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci 23, 1169–1178.
Henneman E, Somjen G & Carpenter DO (1965). Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28, 599–620.
Panizza M, Nilsson J, Roth BJ, Basser PJ & Hallett M (1992). Relevance of stimulus duration for activation of motor and sensory fibers: implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr Clin Neurophysiol 85, 22–29.
Tomlinson SE, Burke D, Hanna M, Koltzenburg M & Bostock H (2010). In vivo assessment of HCN channel current (Ih) in human motor axons. Muscle Nerve 41, 247–256.
Trevillion L, Howells J, Bostock H & Burke D (2010). Properties of low-threshold motor axons in the human median nerve. J Physiol 588, 2503–2515. http://jp.physoc.org/content/588/13/2503.long