
Physiology News Magazine
When does more give less in the olfactory system?
Features
When does more give less in the olfactory system?
Features
Written by Johannes Reisert and Alan Gelperin
Monell Chemical Senses Centre, Philadelphia, PA, USA
https://doi.org/10.36866/pn.86.18
Our senses are exposed continuously to sensory stimuli from our surroundings. We can choose to close our eyes or ears, avoid touch or move away from hot or cold, and we can change voluntarily the perceptual quality of stimuli to some extent (for example, the Doppler effect in audition created by moving quickly towards or away from a sound source or defocusing our eyes to blur an image).
Recent results suggest a mechanism by which an olfactory percept can be changed by altering the stimulation rate of the olfactory system due to voluntarily changing breathing or sniffing patterns.
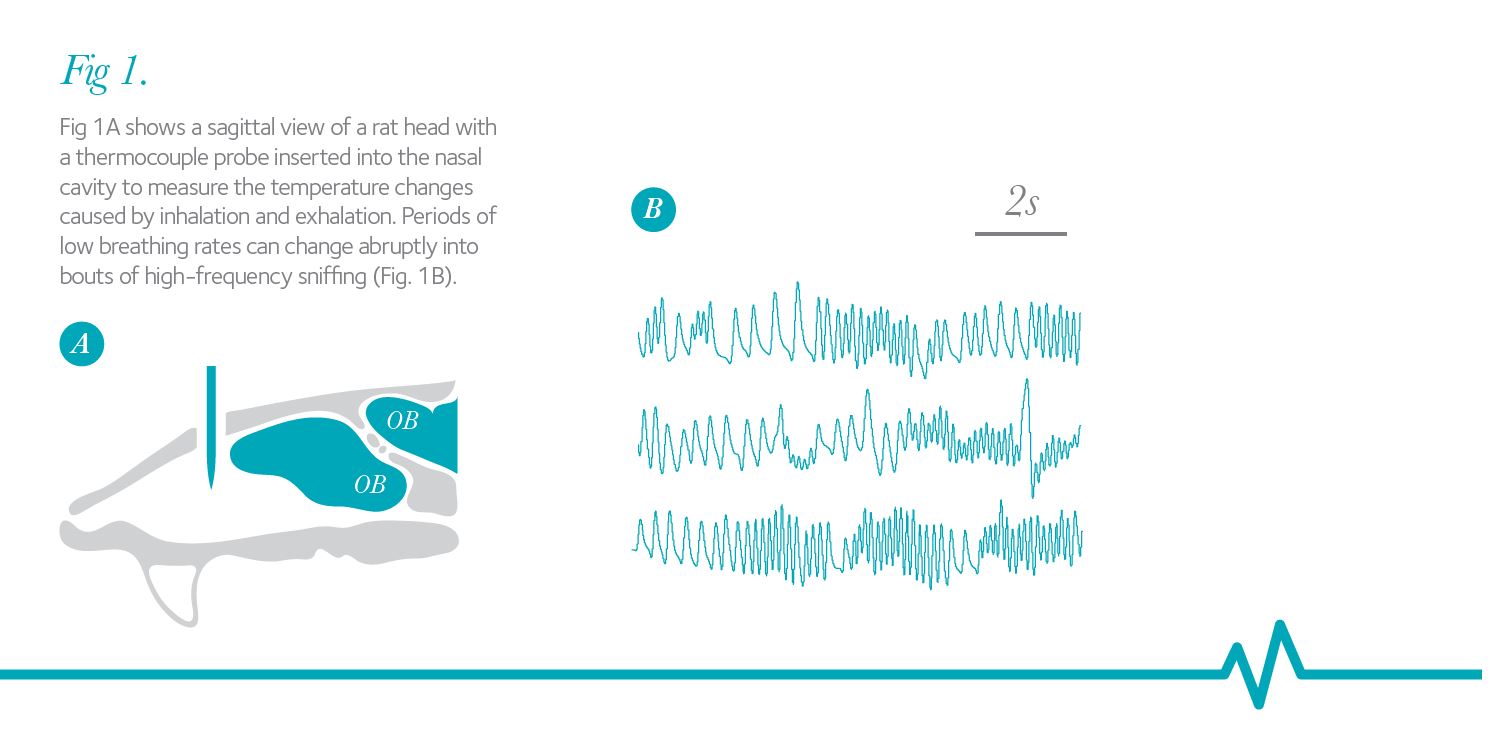
The sense of smell begins with the inhalation of odorants into the nasal cavity. Volatile odorant molecules are carried by the air into the nasal cavity to activate olfactory receptor neurons (ORNs) embedded in the olfactory epithelium (Fig. 1A). Loss of access to the nasal cavity results in reduced olfaction, a phenomenon experienced by reduced flavour perception during a cold. Inhalation is under voluntary control and can change both in frequency and in the volume of inhaled air. Until recently, it was unknown whether and how ORNs in the nasal cavity and second order neurons, mitral cells in the olfactory bulb, encode for such inhalation-based changes in the stimuli. Recent work is beginning to clarify how these changes in the pattern of stimulus delivery to the olfactory epithelium affect responses of both ORNs and mitral cells. In rodents and also in human subjects odour-guided decisions can be based on the information gleaned from a single sniff.
Rodents can vary their breathing (sniffing) frequency from 2 Hz at rest to up to 12 Hz during high-frequency sniffing bouts. Figure 1A shows a sagittal view of a rat head with a thermocouple probe inserted into the nasal cavity to measure the temperature changes caused by inhalation and exhalation. Periods of low breathing rates can change abruptly into bouts of high-frequency sniffing (Fig. 1B), thus changing the delivery of odorant to the olfactory epithelium and the stimulation patterns of ORNs equally abruptly. This active olfactory exploration has been implicated in such diverse functions as directing odorant flow to different parts of the olfactory epithelium, promoting behavioural odorant discrimination, enhancing discovery of new odorants and adaptive filtering of olfactory information.
When mice are asked to perform a simple behavioural discrimination task between very dissimilar odorants, they typically require 250 ms of odorant sampling time, the equivalent of one to three high-frequency sniffs, to determine the identity of the odorants. When confronted with a task to discriminate very similar odours, mice must be forced to extend their odour sampling time to achieve a high level of accuracy. When allowed to make their own decisions about odour sampling time, mice spent the same amount of time sampling odours independent of the difficulty and therefore made less accurate odour discriminations as task difficulty increased (Rinberg et al. 2006). The rapid (few sniffs) decisions mice make when free to do so may relate directly to the decrease in information content of sensory input with sniff number at high sniff sampling rates. On the other hand, the sensory information content clearly does not fall to zero after a few sniffs, as forced prolongation of sniff sampling leads to higher accuracy of odorant discrimination.
But how do ORNs respond to such repeated stimulations at varying frequencies and are those responses dependent on the odorant concentration? Olfactory signal transduction begins with the binding of odour molecules to odorant receptor proteins (ORs) located in the membranes of olfactory cilia and ultimately leads, via a G protein-coupled biochemical cascade and opening of transduction channels (for review see Kleene, 2008), to the generation of action potentials (APs) that are carried to the olfactory bulb via olfactory axons.
The suction pipette recording technique (Lowe & Gold, 1991) can be used to record simultaneously the odorant-induced receptor current and APs produced by ORNs.
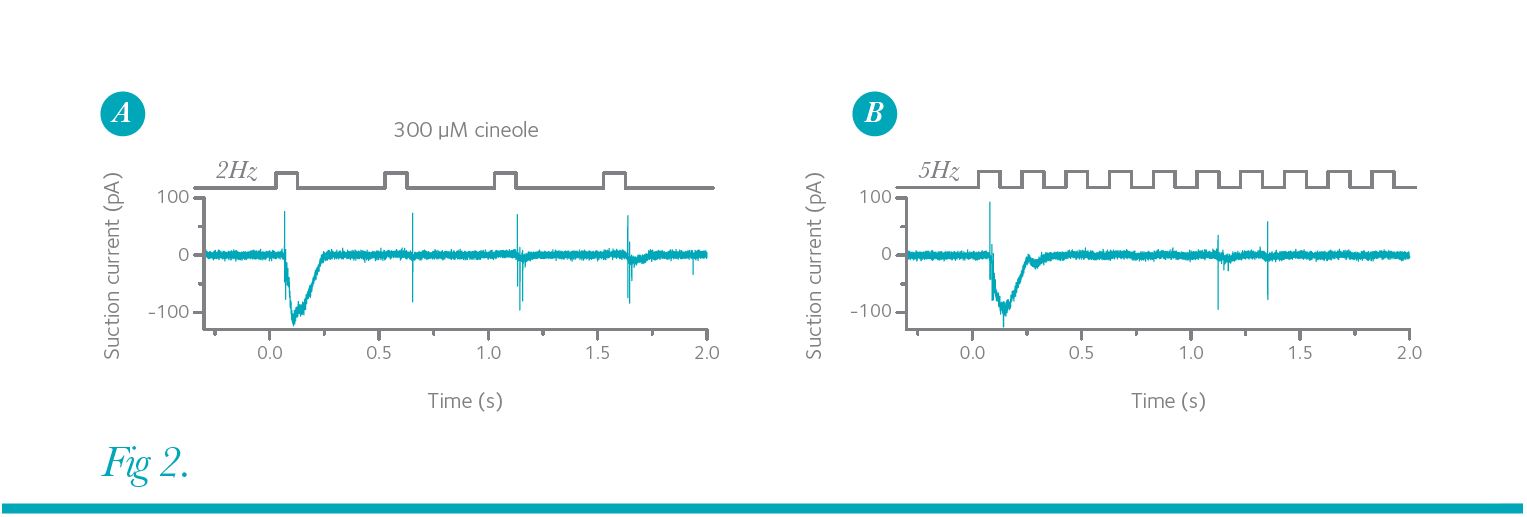
Figure 2A shows such a recording, in which a mouse ORN was exposed repeatedly to the odorant cineole at a frequency of 2 Hz.
The first exposure generated a large receptor current with only a few APs fired during the rising phase of the receptor current. The AP train was cut short because the AP amplitude collapsed quickly (see also responses to the third and fourth stimulation) due to inactivation of voltage-gated Na+ channels during strong depolarizations. Subsequent stimuli reliably generate short bursts of APs upon each stimulation and the repeated application of cineole is faithfully conveyed to the olfactory bulb. This is not the case when the stimulation frequency is increased to 5 Hz (Fig. 2B). After the first odorant exposure, the ORN only generated APs sporadically to subsequent stimuli and hence this ORN continued to provide information, albeit less precisely, for each stimulation.
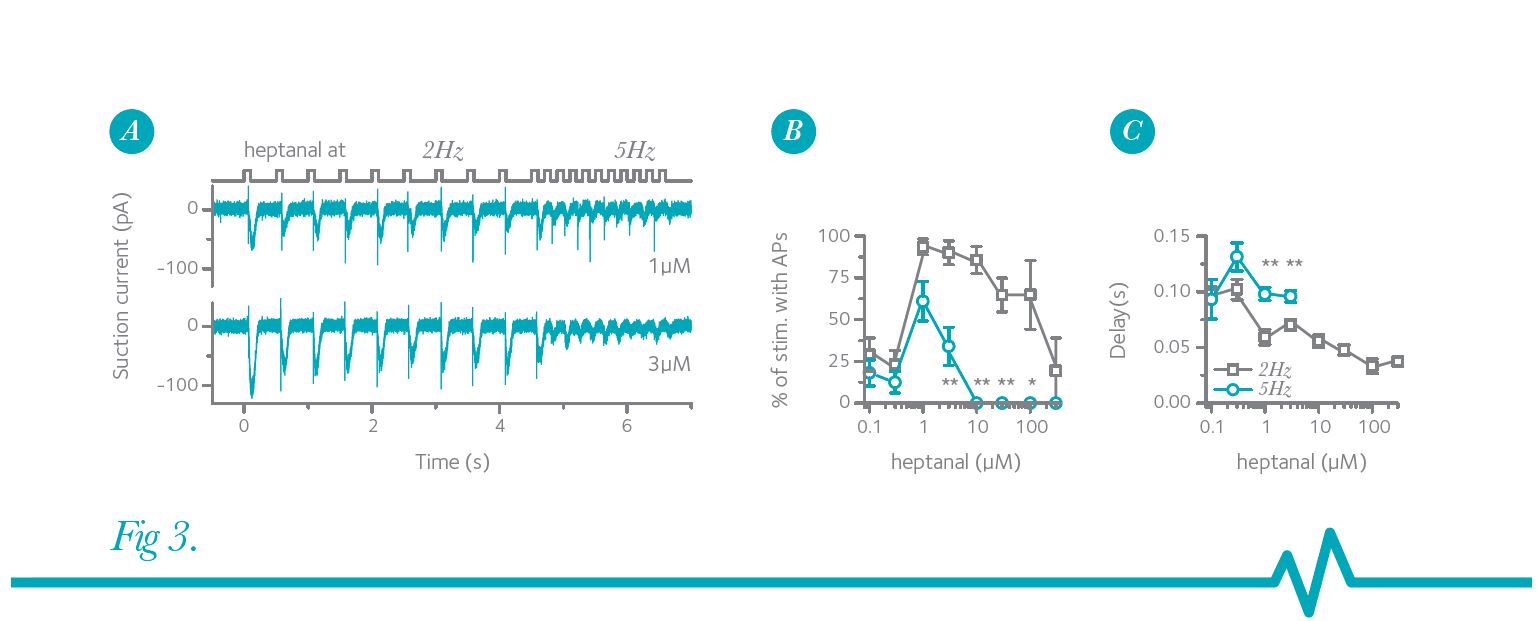
Furthermore, this stimulation-frequency-dependent reduction of AP firing is dependent on the odorant concentration. In a modified stimulus protocol designed to simulate the rapid switch from low- to high-frequency sniffing, an ORN was exposed to heptanal first at 2 Hz followed by 5 Hz stimulations (Fig. 3) (Ghatpande & Reisert, 2011). For these though the ORN still faithfully responded during the 2 Hz stimulation. Thus, from the view of this particular ORN and what information is being relayed to the bulb, an increase in stimulation frequency and concentration greatly reduced the information content. Figure 3B shows that ORNs can respond with AP firing to 2 Hz odorant exposures with nearly 100% accuracy at intermediate odorant concentrations and only begin to fail at high concentrations. In contrast, accuracy never reached levels higher than 60% at 5 Hz stimulation, and failed entirely at concentrations higher than 10 µM heptanal.
Thus, the presence of or change to a higher heptanal concentration might still be relayed to the brain, although through an entirely different set of ORNs and thus mitral cells.
In conclusion ORNs do not simply report the presence and concentration of an odorant but they also modulate their output depending on the stimulation frequency. These results give further impetus to the need to parse olfactory adaptation at the level of intact organisms between peripheral sensory events and central processes (Dalton, 2000)
A requirement for APs to be generated during a subsequent stimulation is that the receptor current from the previous stimulation has terminated. This allows the ORN to hyperpolarize so that voltage-gated Na+ channels can recover from inactivation. A mutation in the cyclic nucleotide-gated channel that causes subtle prolongations of the receptor current (Song et al. 2008) further reduces the likelihood for AP generation during repetitive stimulation with an equivalent reduction in odorant-induced local field potentials recorded from the olfactory bulb. Also, recordings from mitral cells of artificially ventilated mice or rats (to experimentally control the ORN stimulation frequency) showed that an increased odorant exposure rate leads to reduced firing of mitral cells although, unlike the case in ORNs, mitral cell firing does not seem to be entirely suppressed at high stimulation frequencies (Bathellier et al. 2008; Carey & Wachowiak, 2011). A possible reason for the difference in ORN vs. mitral cell responses might be the high convergence of more than 1000 ORNs expressing the same OR to around 25 mitral cells receiving input in a single glomerulus.
So why sniff if it apparently leads to loss of information? A tantalizing interpretation of these results is that it provides the animal with the possibility to selectively suppress its responses to continuously present odorants by voluntarily increasing its odorant sampling frequency (as in Fig. 3A, 3 µM trace). This mechanism has been described as adaptive filtering (Verhagen et al. 2007). A novel odorant experienced during a high-frequency sniff that activates previously unstimulated ORNs can still generate responses, at least during the first odorant exposure (see Fig. 2A). This can be sufficient for odorant identification as the discrimination task only has to be performed in the presence of a background activity that had been reduced due to previous high-frequency sniffing. While adaptive filtering seems to function at the ORN and bulb level, where less AP firing could indeed mean more, it remains to be seen how such a frequency-dependent modulation of ORN activity contributes to odorant discrimination at a behavioural level.
Acknowledgments
The work was supported by a Morley Kare Fellowship and an NIH grant (DC009613). This material is based upon work supported by the US Army Research Office (grant number W911NF-11-1-0087). The authors would like to thank Drs K. Field and G. Lowe for fruitful suggestions and discussion of the manuscript.

