
Physiology News Magazine
When oxygen is not enough: why is fetal growth decreased at high altitude?
Fetal growth is decreased and infant mortality is increased at high altitude (>2700 m). This means that natural selection is acting against mothers and babies that cannot adapt to high-altitude hypoxia, and perhaps selecting for attributes that protect against chronic hypoxia. Comparison of adapted (native) versus non-adapted (migrant) populations is uncovering the mechanisms that regulate fetal growth in an adverse environment and suggesting new avenues for research
Features
When oxygen is not enough: why is fetal growth decreased at high altitude?
Fetal growth is decreased and infant mortality is increased at high altitude (>2700 m). This means that natural selection is acting against mothers and babies that cannot adapt to high-altitude hypoxia, and perhaps selecting for attributes that protect against chronic hypoxia. Comparison of adapted (native) versus non-adapted (migrant) populations is uncovering the mechanisms that regulate fetal growth in an adverse environment and suggesting new avenues for research
Features
Stacy Zamudio
Department of Obstetrics, Gynecology and Women’s Health, New Jersey Medical School, Newark, NJ, USA
https://doi.org/10.36866/pn.76.7

Human birth weight is the result of an evolutionary process known as stabilizing selection. In stable environments there is negative selection against the high and low extremes of birth weight, resulting in mean birth weights that approximate the optimal birth weight, i.e. that at which post-natal mortality risk is lowest. Humans are unique among mammalian species in having complications of pregnancy such as preeclampsia and intrauterine growth restriction (IUGR). Amongst other primates, abnormal fetal growth is extremely rare. IUGR costs billions per year in medical and long-term social costs (e.g. physical handicaps, diminished cognitive abilities). These costs accrue throughout life: IUGR infants have an increased risk of developing cardiovascular and metabolic disease later in life (Gluckman et al. 2008). It is therefore imperative to find out why a significant number of human pregnancies are afflicted with abnormal fetal growth.
Human residence at high altitude (HA, >2700 m) is a natural experimental model for the study of IUGR. The distribution of birth weights is left-shifted and ~12% of infants are classified as IUGR versus 2% at sea level. This disadvantage was first noted in 1639, by Father Antonio de Calancha, who wrote of life in the Andes:
‘In Potosi, all children born of Spanish parents died either at birth or within a fortnight thereafter, because the great cold and freezing air would kill them; the mothers used to leave in order to give birth in the neighbouring valleys and until their child was more than a year old the mothers would exile themselves from this city.’

Note that it is Spanish parents that are affected, as the altitude-adapted natives did not have a similar problem; indeed there is a gradient of altitude-associated growth restriction such that the longest resident high-altitude populations (e.g. Tibetans) have the least decrement in fetal growth for a given altitude. La Paz, Bolivia, at 3000–4000 m in elevation (above) is one of the most populous high-altitude cities in the world and home to considerable numbers of migrants and natives to HA (below). La Paz draws its native population from all over the 4000 m plateau known as the altiplano (also below, Lake Titicaca in the heart of the altiplano).


This renders it ideal for studies of pregnancy physiology to discern what mechanisms cause decreased fetal growth. Or, from a reverse engineering perspective, one can ask, what is it that buffers HA natives from altitude-associated IUGR?
Both experimental animal and human studies have shown that it is lowered oxygen tension (PO2) rather than nutritional status or other risk factors that is ultimately responsible for the decrease in birth weight. A recent study in chick embryos of low-altitude origin shows unequivocally that the ~38% decrease in fetal oxygen tension is associated with a 45% decrease in fetal growth, which is completely reversed by addition of oxygen (Giussani et al. 2007). However, in this experimental model oxygen diffuses through the eggshell directly from the air, whilst mammals have a complicated organ, the placenta, mediating between mother and fetus. In the human we were surprised to find that despite a ~40% reduction in maternal PO2, there was only a 10% decrease in fetal PO2 in both the adapted natives and recent migrants (Europeans) to HA. However, the altitude-associated fall in birth weight was >16% in migrants versus 6% in adapted natives (Postigo et al. 2009). Oxygen was not the direct cause, as we found that fetal O2 delivery was well in excess of fetal O2 consumption, which was similar across all four groups.
Across numerous mammalian species, blood flow (and hence O2 delivery) can decrease by as much as 50% before fetal growth suffers (Carter, 1989). Our HA fetuses defended their O2 supply by increased maternal and fetal oxygen-carrying capacity, increased fetal extraction of O2, and improved oxygen transfer from mother to fetus and from fetal blood to fetal tissue via the Bohr effect. These adaptations should have compensated for the ~30% fall in fetal blood flow in both Europeans and Andeans at 3600 m (Fig. 1), but it did not.
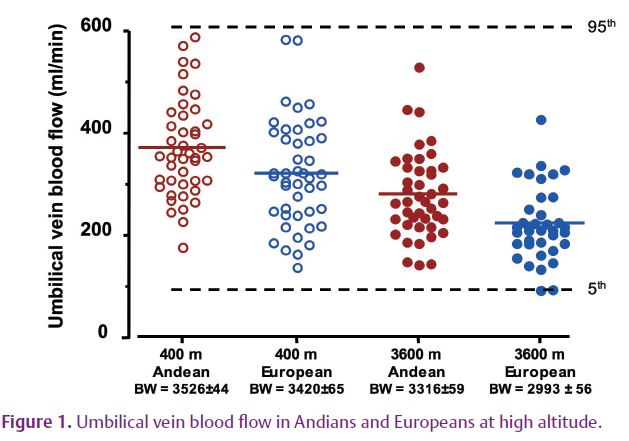
In theory, reduction in blood flow could diminish flow-mediated transport of glucose and amino acids. However, the distribution of fetal blood flows within each group is well within the 5th–95th percentiles for near-term blood flow according to sea level reference values (Fig. 1) (Acharya et al. 2005). The important conclusions in this figure are (1) birth weight (BW) and blood flow are greater in Andeans than Europeans regardless of altitude; and (2) there is considerable overlap in the range of values within each group. The mean values, indicated by the horizontal bar in each scatterplot, range from the 50th to 75th percentile of the sea level reference values. So why is birth weight still reduced, and what accounts for the population difference?
There are several possible answers. First, Native Americans appear to have greater birth weights than Europeans in a variety of environments. Moreover they share ancestral origins with Tibetans. So it is possible that both Tibetans and Andeans are protected against altitude-associated IUGR by virtue of shared genetic ancestry. For example, both Tibetans and Andeans show evidence of increased blood flow mediated via nitric oxide (Beall et al. 2001). Second, Tibetans have been at altitude longer and show a smaller decrease in fetal growth than Andeans, thus additional, possibly genetic, adaptations cannot be ruled out. Third, the pattern of increased Andean birth weight at any altitude may reflect a more rapid accommodation to altitude than was previously suspected. Antonio de Calancha reported that for 50 years no Spanish neonate survived at HA, but they surely survived later in the colonial period. A rapid adaptation of this type could be due to epigenetic modification of the placenta. Such changes have already been shown in pregnancy pathologies (Chelbi & Vaiman, 2008) and were invoked by Giussani and colleagues when their experiments in chicks showed that the HA embryos grew bigger when gestated at sea level (Giussani et al. 2007). Finally, there is the recently advanced theory of metabolic reprogramming which posits a hypoxia-induced, reversible switch to hypometabolism, which spares oxygen at the cellular level, but at the cost of increased glucose consumption (Aragones et al. 2009). This would be consistent with the maintenance of fetal oxygenation at HA, and with our most recent data showing markedly decreased glucose transfer and consumption in the HA fetus. In this last case, decreased fetal growth represents an exquisite balancing act by the placenta to promote oxygen diffusion to the fetus while at the same time limiting glucose delivery and thereby down-regulating fetal growth. It is thus the placenta that may be programming the fetus, which in turn may be responsible for the long-term increase in the risk of chronic disease that IUGR infants incur as a cost of their intrauterine survival.
References
Acharya G, Wilsgaard T, Rosvold Berntsen GK, Maltau JM & Kiserud T (2005). Reference ranges for umbilical vein blood flow in the second half of pregnancy based on longitudinal data. Prenat Diagn 25, 99–111.
Aragones J, Fraisl P, Baes M & Carmeliet P (2009). Oxygen sensors at the crossroad of metabolism. Cell Metab 9, 11–22.
Beall CM, Laskowski D, Strohl KP, Soria R, Villena M, Vargas E, Alarcon AM, Gonzales C & Erzurum SC (2001). Pulmonary nitric oxide in mountain dwellers. Nature 414, 411–412.
Carter AM (1989). Factors affecting gas transfer across the placenta and the oxygen supply to the fetus. J Dev Physiol 12, 305–322.
Chelbi ST & Vaiman D (2008). Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol Cell Endocrinol 282, 120–129.
Giussani DA, Salinas CE, Villena M & Blanco CE (2007). The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol 585, 911–917.
Gluckman PD, Hanson MA, Cooper C & Thornburg KL (2008). Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359, 61–73.
Postigo L, Heredia G, Illsley NP, Torricos T, Dolan C, Echalar L, Tellez W, Maldonado I, Brimacombe M, Balanza E, Vargas E & Zamudio S (2009). Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. J Physiol 587, 693–708. http://jp.physoc.org/content/587/3/693.long
