
Physiology News Magazine
Why do physiologists work on snails? A personal perspective
A mighty brain in a simple organism
Features
Why do physiologists work on snails? A personal perspective
A mighty brain in a simple organism
Features
Paul Benjamin
Sussex Neuroscience, School of Life Sciences, University of Sussex, UK
https://doi.org/10.36866/pn.99.27
By the early 1970s it became clear that snails are excellent subjects for physiologists interested in relating neuronal electrical properties to behaviour. Individual large neurons (up to 150µm in diameter) were identified in the central ganglia of the snail brain (Fig. 1) as parts of defined behavioural circuits and their synaptic connections determined by paired intracellular recordings. Since then physiologists working on snails have made significant progress in solving a wide range of fundamental physiological problems from membrane biophysics and peptidergic signalling, through motor pattern generation to learning and memory. I have chosen examples that illustrate some of the advances that have been achieved by exploiting snails.

Not such a simple system
In my choice of the pond snail, Lymnaea, I was strongly influenced by a paper published in Science by Dennis Willows in 1967 demonstrating that stimulation of single giant neurons in sea slugs reliably elicited sequences of muscular movements underlying rhythmic swimming. It made me believe that if I could elucidate the neural circuits (‘wiring’ diagram) underlying examples of these stereotyped behaviours then I could understand how the brain works. This assumption turned out to be extremely naïf and I spent the next thirty years trying to understand how the ‘simple’ brain of Lymnaea is organized (Benjamin, 2008).
Multi-channel model of the nerve cell membrane
Early work on snails focussed on the biophysics of snail giant neurons whose large size facilitates multi-electrode impalement. Two novel types of potassium current, distinct from the classical Hodgkin-Huxley delayed rectifier, were discovered that were of general significance.
One type is the voltage-sensitive potassium A current, sometimes called the transient potassium current. This current is activated at the sub-threshold region of the membrane potential and helps to control the frequency of repetitively firing neurons. Although it is largely inactivated near the resting potential and completely inactivated during the action potential, the inactivation is removed by the after-hyperpolarization that normally follows an action potential. The A current is active for a short period after each action potential and delays the return of the membrane potential to spike threshold. This determines the duration of the inter-spike interval and thus the frequency of firing.
The other is the calcium-dependent potassium current. This type of ion current is also involved in controlling the pattern of firing of snail neurons. Some neurons that fire spontaneously do not fire at regular intervals but instead generate regular bursts of action potentials, separated by hyperpolarizations of the membrane potential. During the spiking phase of bursting neurons there is an influx of calcium into the neurons that progressively increases the intracellular calcium concentration. This activates the calcium-sensitive potassium current resulting in a delayed hyperpolarization of the membrane potential and a consequent cessation of firing. The calcium-dependent current thus contributes to the ‘silent’ phase of bursting neurons.
The electrogenic sodium pump in neurons was first investigated in the 1960s by Roger Thomas in the snail Helix aspersa. An active ion transporter, the sodium-potassium ATPase or sodium-potassium pump, mediates the pumping of sodium ions out of and potassium ions into the neuron to maintain the ion concentration gradients across the cell membrane. The stoichiometry of the ATPase, that determines the transport ratio for sodium and potassium, is 3:2 and so three sodium ions are transported out of the cell for every two potassium ions that are transported inwards. As a result the pump produces a net outward current. This kind of pump is said to be electrogenic because its activity causes the neuron to hyperpolarize and thus contributes to the setting of the resting potential.
Why so many neurotransmitters?
How neurons communicate which other is still a major question in physiology. It has been made more complex by the realisation that large numbers of neuropeptides modulate the basic neuron to neuron communication systems that use ‘fast’ transmitters such as acetylcholine and L-glutamate. It has been suggested that neurons are bathed by a ‘chemical soup’ of multiple peptide transmitters that indirectly modulate the more direct local inhibitory and excitatory synaptic pathways between neurons. In the snail, neuropeptides are distributed throughout the central nervous system, and although there are about 100 different types, they can be located in identified neurons and their functions investigated by a combination of molecular and electrophysiological techniques. A major advance in understanding the complexity of peptide signalling, was the use of mass spectrometry to identify multiple neuropeptides in a single neuron; for example in neurons expressing the FMRFamide gene a total of 13 different FMRFamide-related peptides were identified. A pair of identified cardio-excitatory motoneurons were found to co-release five different peptides, each with a distinct function in heartbeat control. This work revealed the sophistication of peptidergic signalling in the nervous system controlled by a single gene. The neuropeptides involved in heart-beat control target G protein coupled receptors (GPCRs) and use different second messenger pathways. For instance, FMRF/FLRFamide increase the rate and amplitude of heart beats by mobilising the inositol phosphate pathway whereas the co-released EFLRamide peptides are mediated by a cyclic AMP-mediate pathway and these produce more prolonged excitatory effects on heartbeat compared with FMRFamide. FMRFamide, however, can elicit excitatory effects on snail neurons without activating G-proteins. FMRFamide application directly gated sodium channels with pharmacological properties similar to that of mammalian epithelial sodium channels (ENaCs): they were highly selective for sodium, blocked by amiloride and were insensitive to blockers of other sodium ion channel types. This was the first type of peptide-gated ion channel to be described in any system (Cottrell, 1997)!
How are neural networks organized?
Classifying neurons into types eg motoneurons, interneurons and sensory neurons is a classic way of trying to understand the organization of neural circuits. We investigated whether this method of classification is still useful in a snail feeding circuit where about 100 neurons control the rhythmic feeding movements that the snail uses to ingest food. This analysis of function is possible because we can identify all the neurons and their synaptic connections in Lymnaea (Benjamin, 2012). Rhythmic motor behaviour in feeding and other behaviours like respiration are generated by central pattern generators (CPGs) and the emphasis has been on identifying the basic rhythm generating machinery, identifying the interneurons that form the CPG networks and describing their firing patterns and synaptic connectivity. A first was the isolation and growing of the three neurons of the respiratory CPG in culture to form a functional reconnected network to recapitulate the rhythmic firing pattern seen in the intact ganglia. Subsequently it was realized that the feeding CPG was controlled by several types of modulatory interneuron, which form a part of a highly interconnected network with properties that underlie flexible responses to internal and external stimuli.
Recent work indicates that many of the feeding neurons are multifunctional and that properties traditionally attributed to one class of neurons are distributed across several neuronal types. For instance, feeding motoneurons play a role in rhythm generation via their coupling to CPG interneurons. Many different neurons are involved in the initiation of feeding due to the wide neuronal distribution of chemosensory inputs that drive feeding. The epitome of multi-functionality is a single interneuron that as well as being part of the rhythm-generating CPG circuit is also involved in switching of behaviour from quiescence to feeding. It mediates the effects of hunger and satiety as well. It fires continuously in a single spiking pattern to inhibit feeding when the animal is satiated but fires in bursts to be part of the CPG pattern-generating mechanism when the snail is hungry.
This ‘distributed’ organization of network function has been successfully modelled in a recent computational study. This use of neurons for more than function may be a type of ‘economy’ measure in the snail networks with a small numbers of neurons available but on the other hand it is also observed in other animals including vertebrates where much larger numbers of neurons are involved.
Snails are quite brainy: how do they store memories and why do they have memory lapses like us?
Snails have been used extensively for studying the neural mechanisms underlying a type of associative learning (reward classical conditioning) first described by Pavlov in dogs. Reward classical conditioning of feeding is performed in Lymnaea by pairing amyl acetate (the CS, conditioned stimulus) with sucrose (the US, or unconditioned stimulus). Amyl acetate is not a feeding stimulus in naïve snails, unlike sucrose, but after CS+US training the snail perceives it as food. Remarkably, a single training trial leads to a long-term memory that persists for up to 3 weeks. As well as these simple forms of associative learning, snails are capable of other forms of learning whose features are similar to those found in vertebrates. For instance stimulus generalization (responding to related stimuli), goal tracking (moving towards the reinforcing food stimulus) and context dependency (increased learning in a novel environment) have been demonstrated in Lymnaea.
By taking advantage of the ability to record from identified neurons in the snail, it is possible to record electrical correlates of memory formation in single neurons of the feeding network following single-trial reward chemical conditioning. Mechanistic studies were carried out on single neurons and their synaptic connections. Changes at multiple synaptic sites within the feeding network were found, yielding a surprisingly complex picture of the mechanisms involved in learning and memory. Increases in strength of excitatory synapses that facilitate interneuron and motoneuron responses to the CS have been found. Conditioning also results in a reduction in inhibitory synaptic inputs to the feeding CPG, also directly contributing to the conditioned response. The previously favoured model for explaining associate conditioning in molluscs involved restriction of changes to a single site. Pioneering work on snails has shown that non-synaptic neuronal changes such as increases in excitability also play an important role in learning. These result from a learning-induced change in intrinsic ion currents. An example of this comes from our own work using one-trial chemical conditioning of feeding. The modulatory interneurons, the Cerebral Giant Cells (CGCs) are persistently depolarized by about 10 mV between 16-24 hours after conditioning. This increases the strength of post-synaptic responses to CGC stimulation by a process that involves an increase in the intracellular calcium concentration in the proximal dendritic processes of the CGCs. The conditioning–induced depolarization is due to an increase in the amplitude of a cyclic AMP-sensitive persistent sodium current.
An intriguing recent aspect of our work on learning and memory in snails has revealed specific time points after training when temporary lapses in memory expression occur (Marra et al. 2013). These coincide with transitions between different molecularly-defined phases of memory. Reports of memory lapses during memory consolidation are widespread. They have been observed in many types of organism including human subjects raising general questions about function. By application of novel sensory stimulation during the lapses (but not at other time points), we found that memory consolidation becomes vulnerable to these ‘disturbing’ stimuli and leads to the blocking of the subsequent progress of consolidation. We have speculated that lapses represent choice points that allow the memory trace to be expressed adaptively according to the variety of novel external stimuli that the animal is exposed to in the environment. More recently we have shown that the initial memory trace can be replaced by another memory trace if another type of training is carried out at the lapse point, suggesting that the consolidation of memory is an extremely adaptive process.
How does a snail decide what to do next?
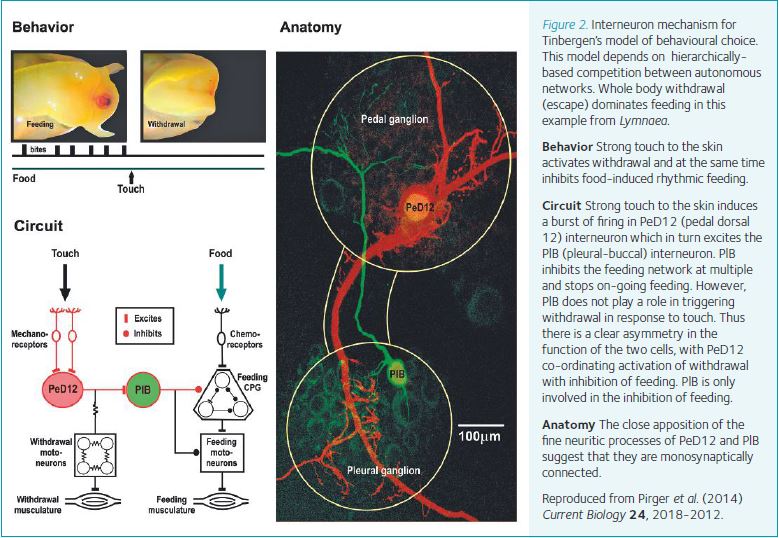
We have to make decisions about what to do next on a moment by moment basis. Often these decisions are between incompatible behaviours. Early in the field of neurophysiology and behaviour, the Nobel Prize-winning ethologist, Niko Tinbergen (1951), suggested that choices between incompatible behaviours involve hierarchically-based inhibitory interactions between autonomous neural circuits providing priority to actions more important for survival. Although this model is still very influential there is little supporting evidence for it in either vertebrates or invertebrates. In our Lymnaea snail system, we were are able to provide direct electrophysiological evidence for this model taking advantage of the ability to identify neurons responsible for behavioural choice (Pirger et al. 2014). As predicted by the Tinbergen model, defensive escape behaviour (withdrawal into the shell) takes precedence over feeding. By recording neurons from the feeding and escape networks, we found no direct synaptic connections between the interneuronal and motoneuronal elements that generate the two behaviours. Instead, we discovered a novel interneuronal pathway that when activated by an aversive stimulus (a strong poke), triggers escape but at the same time inhibits feeding. This asymmetrical inhibitory interneuronal pathway allows one behaviour to dominate the other (Fig. 2). This is the first cellular level analysis of the original Tinbergen’s hypothesis proving the existence of a hierarchical decision-making process at the level of a simple circuit.
References
Benjamin PR (2008). Lymnaea. Scholarpedia. 3, 4124. http:www.scholarpedia.org/article/Lymnaea.
Benjamin PR (2012). Distributed network organization underlying feeding behavior in the mollusk Lymnaea. Neural Syst. Circuits 2: 4. DOI: 10.1186/2042-1001-2-4
Cottrell GA (1997). The first peptide-gated ion channel. J. Exp. Biol. 200, 2377-2386.
Marra, V, O’Shea M, Benjamin PR & Kemenes I (2013). Susceptibility of memory consolidation during lapses in memory recall. Nat. Commun. 4, 1578. DOI: 10.1038/ncomms 2591
Pirger Z, Crossley M, Laszlo, Z, Naskar, S, Kemenes G, O’Shea M., Benjamin, PR & Kemenes I (2014). Interneuronal mechanism for Tinbergen’s Hierarchical model of behavioral choice. Curr. Biol. 24, 2018-2024..
