Physiology News Magazine
Work against gravity: in search of the molecular switch for mechano-regulated muscle plasticity
Prolonged reductions in weight-bearing muscle activity during bedrest or spaceflight cause dramatic loss in strength due to wasting of postural muscle. Astonishingly little is known concerning the mechano-sensory pathways which underpin this response. Our recent work addresses the process of mechano-transduction by using somatic transgenesis and altered loading to modify the putative mechanoreceptor
Features
Work against gravity: in search of the molecular switch for mechano-regulated muscle plasticity
Prolonged reductions in weight-bearing muscle activity during bedrest or spaceflight cause dramatic loss in strength due to wasting of postural muscle. Astonishingly little is known concerning the mechano-sensory pathways which underpin this response. Our recent work addresses the process of mechano-transduction by using somatic transgenesis and altered loading to modify the putative mechanoreceptor
Features
Martin Flück
Institute for Biomedical Research into Human Movement and Health, Manchester Metropolitan University, UK
https://doi.org/10.36866/pn.78.28

Work-related stimuli are critical for maintenance of muscle function (Loughna & Goldspink 1990). This physiological control is apparent in the dramatic loss of force and metabolic capacity associated with reductions in muscle loading during real or simulated microgravity, and the reversal of these deteriorations with subsequent resumption of load-bearing muscle activity. These responses highlight the instrumental role of mechanical factors in the conditioning of muscle structure and function. In contrast to the accepted clinical importance of this mechano-dependent muscle plasticity and identified mechano-sensible signalling factors (Nader & Esser, 2001) there is a distinct lack of understanding of the signalling mechanisms in vivo.
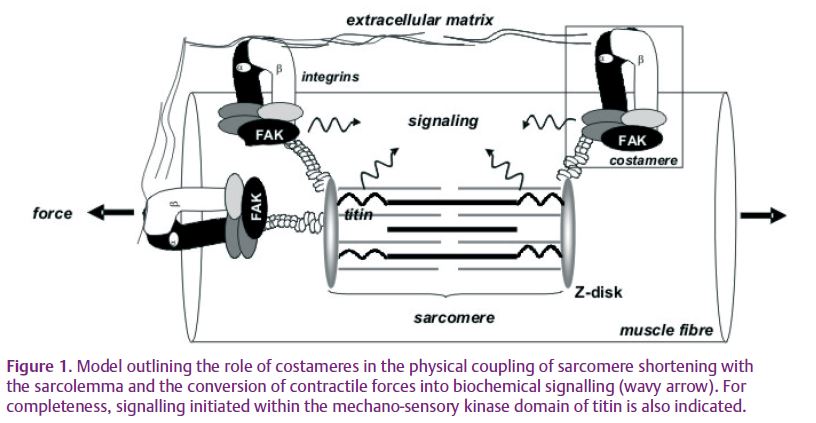
Focal complexes of proteins have been proposed to convert the mechanical factors of contraction into biochemical signalling inside muscle fibres (Fig. 1; Lange 2005; Durieux et al. 2009). This idea is based on the association of focal adhesions with specific signalling molecules in cultured muscle cells (Shyy & Chien, 1997) and the architectural coupling of sarcolemma sites of fibre adhesion (costameres) with sarcomere shortening during muscle contraction (Pardo et al. 1983). The functional involvement of costameres in mechano-transduction and phenotypic control of fully differentiated striated muscle is largely unexplored. This may be because integrin receptors which form the adhesive core of costameres are devoid of enzymatic activity. Indirect observations suggest that mechano-transduction is initiated by strain-induced conformational changes in integrins (Katsumi et al. 2005) and the subsequent coupling to intracellular signalling (reviewed by Durieux et al. 2009). The kinase enzymes involved in this response have, until lately, not been characterized.
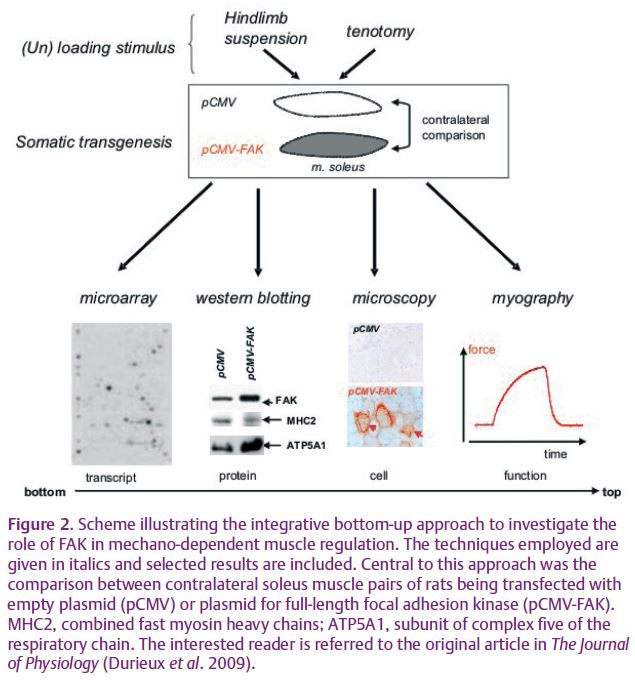
Our recent investigation addressed this topic by focusing on the role of the integrin-associated phosphotransfer enzyme focal adhesion kinase (FAK; Durieux et al. 2009). In cultured cells, FAK is involved in mechano-chemical coupling between mechanical stimulation of integrins and intracellular signal transduction. Phosphorylation of FAK at tyrosine residue 397 (Y397) is considered to reflect activation of FAK and downstream signal transduction (Shyy & Chien, 1997). The role of this signalling molecule in mechano-transduction in intact tissue had so far not been investigated owing to the lethal consequences of FAK inactivation via the germline. Our study addressed this question using somatic technology which allowed overexpression of FAK in fully developed anti-gravitational muscle. The loading state of the muscle was physiologically modulated in a ground-based model of microgravity (hindlimb suspension) and muscle loading (tenotomy; Fig. 2). The monitoring of transcriptional, myocellular and contractile consequences pointed out myofibre transformation with the overexpression of full-length FAK in soleus muscles towards a slow-oxidative phenotype. Competition experiments with the combined overexpression of a FAK inhibitor (FRNK) demonstrated the specificity of FAK-regulated transcript expression. Probing with a native, rather than a constitutively active, FAK protein exposed the post-translational activation of FAK by phosphorylation at tyrosine Y397 as a molecular switch of mechano-regulated muscle plasticity (Durieux et al. 2009).
Our findings compare well with recent findings on the down-regulation of the activation status of FAK during muscle disuse atrophy in humans (de Boer et al. 2007) and FAK-mediated activation of the S6Kinase-dependent pathway (Klossner, 2009). These observations indicate that costamere-mediated mechano-transduction is involved in the anabolic control of muscle mass. It remains to be determined to what degree this mechanism is selective for activation of muscle protein synthesis or whether it controls protein turnover in general.
This possibility was suggested by the mechano-modulated control of expression of the gene ontologies for protein degradation by FAK (Durieux et al. 2009). The outlined experimental approach (see Fig. 2) provides an important step forward towards the investigation of signal integration in a fully developed tissue. The success of the experiments relied on the exceptional capacity of muscle for uptake of naked DNA upon electropulsing, and the monitoring of downstream consequences of overexpression with a combination of classical and modern technologies. We reason that this set-up may be of generic interest to muscle research as it offers a ready, low-cost alternative to labour-intensive and expensive transgenic approaches via the germline. The electro-assisted muscle transfection technique also has the benefit of reducing biological noise by allowing the inclusion of intra-animal, i.e. contralateral, controls in paired muscle groups. This advantage was shown in somatic knock-in experiments exposing the hitherto unknown consequences of mechano-regulated extracellular matrix protein expression in muscle repair (Flück et al. 2008). The transfection tool therefore fills a gap for the researcher wishing to explore mechanistic relationships with a tight budget or timeline,or in animal species which do not lend themselves to efficient transgenic modification with germline technology.
References
de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV & Rennie MJ (2007). The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585, 241–251.
Durieux A-C, D’Antona G, Desplanches D, Freyssenet D, Klossner S, Bottinelli R & Flück M (2009). Focal adhesion kinase is a load-dependent governor of the slow contractile and oxidative muscle phenotype. J Physiol 587, 3703–3717. http://jp.physoc.org/content/587/14/3703.long
Flück M, Carson JA, Gordon SE, Ziemiecki A & Booth FW (1999). Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol 277, C152–C162.
Flück M, Mund SI, Schittny JC, Klossner S, Durieux AC & Giraud MN (2008). Mechano-regulated tenascin-C orchestrates muscle repair. Proc Natl Acad Sci U S A 105, 13662–1367.
Flück M & Hoppeler H (2003). Molecular basis of skeletal muscle plasticity — from gene to form and function. Rev Physiol Biochem Pharmacol 146, 159–216.
Katsumi A, Naoe T, Matsushita T, Kaibuchi K & Schwartz MA (2005). Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 280, 16546–16549.
Klossner S, Durieux AC, Freyssenet D & Flueck M (2009). Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106, 389–398.
Lange S, Xiang F, Yakovenko A, Vihola A, Hackmann P, Rostkova E et al. (2005). The kinase domain of titin controls muscle gene expression and protein turnover. Science 308, 1599–1603.
Loughna PT, Izumo S, Goldspink G & Nadal-Ginard B (1990). Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development 109, 217–223.
Nader GA & Esser KA (2001). Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90, 1936–1942.
Pardo JV, Siliciano JD & Craig SW (1983). A vinculin-containing cortical lattice in skeletal muscle, transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A 80, 1008–1012.
Shyy JY & Chien S (1997). Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol 9, 707–713.