Physiology News Magazine
β-adrenergic receptor desensitisation – a mechanism for post-exercise reductions in cardiac function
Features
β-adrenergic receptor desensitisation – a mechanism for post-exercise reductions in cardiac function
Features
Rob Shave (1), Keith George (2) & Emma Hart (1)
1: Centre for Sports Medicine and Human Performance, Brunel University, Uxbridge, Middlesex, UB8 3PH
2: Research Institute for Sport and Exercise Sciences, Liverpool John Moores University, Liverpool, L3 2ET
https://doi.org/10.36866/pn.69.27

It is widely recognised that skeletal muscle performance is reduced in response to prolonged exercise; whether the same occurs with cardiac muscle is currently a topic of scientific debate. A number of recent studies have demonstrated a modest and transitory impairment of left ventricular (LV) systolic (ejection fraction and the ratio of end systolic volume to pressure) and diastolic (ratios of early to late LV filling and early and late myocardial wall velocities) function after prolonged exercise such as marathon running and Ironman triathlons.
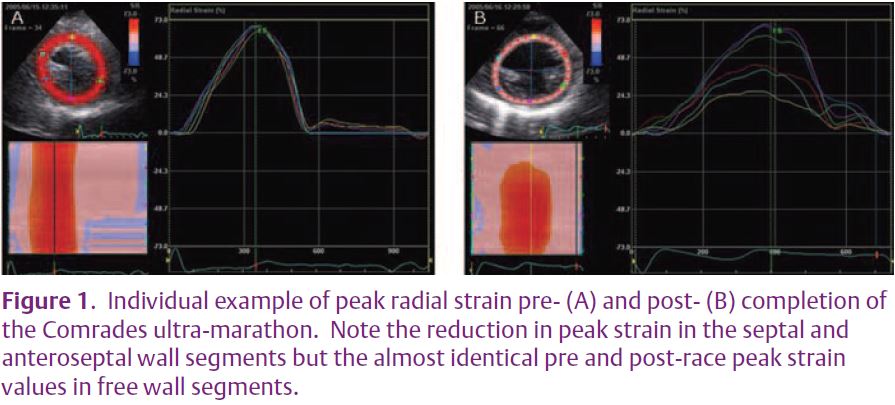
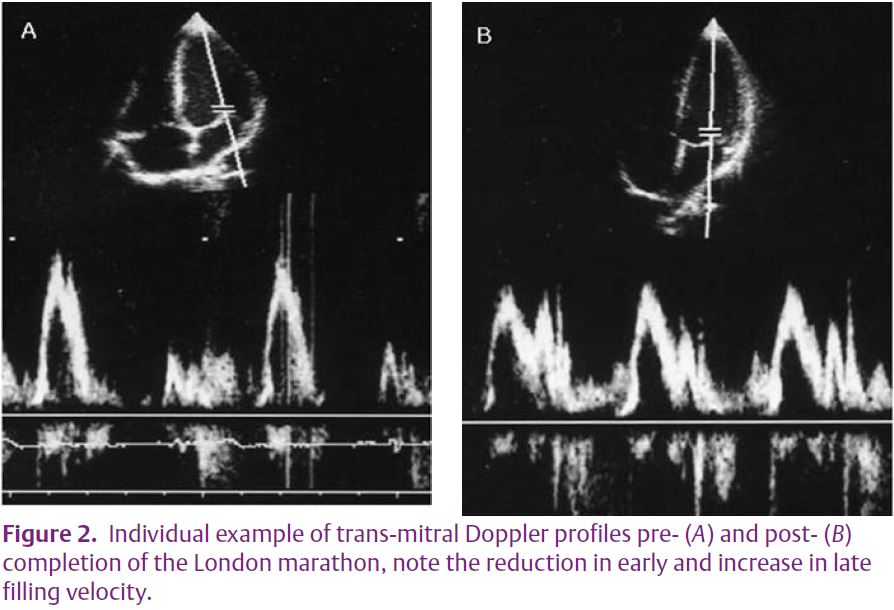
Driven largely by advances in echocardiographic technology, numerous studies have described post-exercise cardiac impairment in increasing detail. The initial studies using 2-dimensional and Doppler measures were improved upon with tissue Doppler and recently 2dimensional speckle tracking. With the advance in technology it has been possible to demonstrate both global and regional changes in systolic function; for example,Rifai et al.(1999) demonstrated a 21% reduction in ejection fraction in addition to segmental wall motion abnormalities. More recently our group have demonstrated regional reductions in peak systolic 2D derived strain and strain rate (longitudinal, circumferential and radial planes) in athletes completing the Comrades ultra-marathon (Fig. 1). Similarly, evidence for reductions in both global and regional diastolic function has been presented (Neilan et al. 2006). The trans-mitral filling ratio and LV flow propagation velocity has been shown to be transiently impaired postexercise (Fig. 2). These global diastolic changes have been mirrored by segmental reductions in diastolic wall velocities, strain and strain rate (George et al. 2006; Neilan et al. 2006).
It is important to recognise the limitations of some of the echocardiographic techniques that have been employed; trans-mitral Doppler, flow propagation velocity and ejection fraction are highly dependent upon loading and heart rate. Accordingly, assessment of these variables pre- to post- exercise warrants caution; however, the regional differences in LV function that have been shown cannot be explained by altered loading or HR. Further, when correlations are examined the changes in LV function following prolonged exercise cannot be fully explained by altered loading or HR. Whilst characterisation of post-exercise alterations in LV function is useful it does not provide a mechanistic explanation.
A number of possible mechanisms for post-exercise changes in LV function have been suggested – including minor injury to the myocardium evidenced by the release of highly cardiac specific markers of injury e.g. cardiac troponin T, impaired sequestration and re-uptake of calcium into the sarcoplasmic reticulum and desensitisation of the β-adrenergic receptors (β- AR). To date, however, empirical evidence supporting a role for any of these mechanisms is limited.
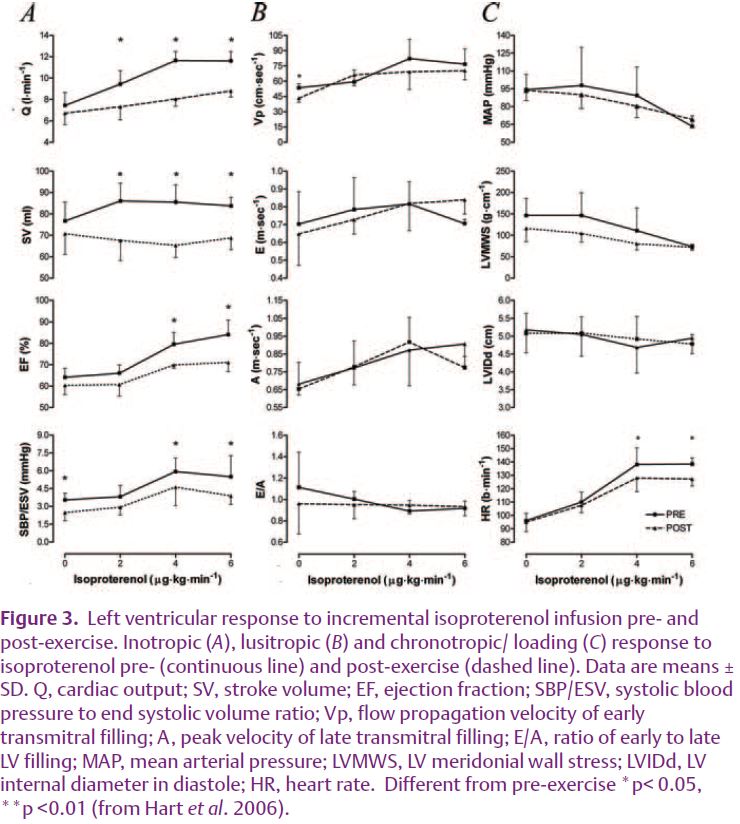
In our recent study we employed pharmacological manipulation to examine the role of β-AR in postexercise changes in cardiac function (Hart et al. 2006). Prior to and following a 4 h bout of rowing exercise we examined the systolic and diastolic response to increasing doses of isoproterenol (a β-AR agonist). In order to partition out pre- to post-exercise changes in vagal tone, glycopyrrolate (a parasympathetic blocking agent) was administered prior to β-AR stimulation. Our data demonstrate that there is a reduction in the chronotropic and inotropic response to β-AR stimulation following 4 h of rowing (Fig. 3). We suggest, therefore, that post-exercise reductions in systolic function are likely related to a de-sensitisation of the β-AR.
Within heart failure patients, a clear link has been demonstrated between elevations in circulating catecholamines and de-sensitisation and down-regulation of the β-AR (Dzimi et al. 1999). It is possible that a similar mechanism may occur following prolonged exercise. Of interest, those participants within our study that demonstrated the greatest elevation in adrenaline following exercise also demonstrated the largest reduction in LV contractility as assessed by ejection fraction (r2=0.96, P<0.05).
The cardiac β-AR subtypes β1 and β2 activate different signalling pathways within the myocyte (Xiao et al. 2004). It is possible that during prolonged exercise the sustained elevations in catecholamines initiates β1-AR mediated apoptosis of the myocyte (Rohrer et al. 1999) which may increase membrane permeability and hence explain the release of cardiac biomarkers such as cardiac troponin T observed following prolonged exercise. Conversely, stimulation of the β2-AR has an anti-apoptotic effect and protects the myocyte against injury (Patterson et al. 2004). Accordingly, de-sensitisation of β1-AR and stimulation of β2-AR during prolonged exercise may occur so as to limit injury to the myocardium. In order to examine the role of the specific β-AR subtypes further work is required.
Over stimulation of the β-AR has been demonstrated to induce hypertrophy, at least in the rat heart (Schafer et al. 2000). Therefore, the sustained exposure of the β-AR to catecholamines during prolonged exercise may also be an initial stimulus for LV hypertrophy often reported in endurance trained athletes. This, however, remains unsubstantiated and warrants further research.
Although desensitisation of β-AR may be responsible for the reductions in LV systolic function post-exercise it is interesting to note that the diastolic response to increasing doses of isoproterenol did not alter following four-hours of rowing (Fig. 3). Accordingly, changes in diastolic function observed following prolonged exercise are likely related to a different mechanism. Previous studies have indicated that in some chronic heart failure models, β-AR de-sensitisation is related to systolic but not diastolic dysfunction due to an up-regulation of the sodium-calcium exchanger (Sato et al. 2004). Consequently, calcium is removed from the myocyte maintaining diastolic function, in spite of an impaired systolic function. Whether this is relevant in the prolonged exercise model is not clear.
The present study provides a mechanistic insight into postexercise alterations in LV systolic function; however, the mechanisms for alterations in diastolic function following prolonged exercise are not fully understood. Further, whether post-exercise changes in LV function translate to a cardiac limitation during prolonged exercise and whether β-AR de-sensitisation is related to LV hypertrophy remains to be elucidated.
References
Dzimiri N. (1999). Regulation of betaadrenoceptor signaling in cardiac function and disease. Pharmacol Rev 51, 465-501.
George K, Oxborough D, Forster J, Whyte G, Shave R, Dawson E, Stephenson C, Dugdill L, Edwards B, & Gaze D. (2005). Mitral annular myocardial velocity assessment of segmental left ventricular diastolic function after prolonged exercise in humans. J Physiol 569, 305-313.
Hart E, Dawson E, Rasmussen P, George K, Secher N, Whyte G, Shave R. (2006) badrenergic receptor desensitization in man: insight into post-exercise attenuation of cardiac function. J Physiol 577, 717-725. Neilan TG, Yoerger DM, Douglas PS, Marshall
JE, Halpern EF, Lawlor D, Picard MH, & Wood MJ. (2006). Persistent and reversible cardiac dysfunction among amateur marathon runners. Eur Heart J 27, 1079-1084.
Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, et al. (2004). Protecting the myocardium: A role for the beta2 adrenergic receptor in the heart. Crit Care Med 32, 10411048.
Rifai N, Douglas PS, O’Toole M, Rimm E, & Ginsburg GS. (1999). Cardiac troponin T and I, echocardiographic [correction of electrocardiographic] wall motion analyses, and ejection fractions in athletes participating in the hawaii ironman triathlon. Am J Cardiol 83, 1085-1089.
Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, & Kobilka BK. (1999). Cardiovascular and metabolic alterations in mice lacking both beta1- and beta2adrenergic receptors. J Biol Chem 274, 1670116708.
Sato M, Gong H, Terracciano CM, Ranu H, & Harding SE. (2004). Loss of beta-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J Mol Cell Cardiol 36, 43-48.
Schafer M, Frischkopf K, Taimor G, Piper HM, & Schluter KD. (2000). Hypertrophic effect of selective beta(1)-adrenoceptor stimulation on ventricular cardiomyocytes from adult rat. Am J Physiol Cell Physiol 279, C495-503.
Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, & Cheng H. (2004). Subtypespecific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci 25, 358365.