 While homeostasis is considered a steady state in physiology, it is by no means static. Instead, it is the sum of a concerted effect of sub-cellular events driven by molecular machines. These machines range from large enzymes to reactive small molecules, all of which exert an effect on the cell to alter its function for better or for worse.
While homeostasis is considered a steady state in physiology, it is by no means static. Instead, it is the sum of a concerted effect of sub-cellular events driven by molecular machines. These machines range from large enzymes to reactive small molecules, all of which exert an effect on the cell to alter its function for better or for worse.
Indeed, the over- or under-activity of these molecular machines can result in disease or injury (e.g. reactive oxygen species (ROS) and inflammation), or in an intended response to applied therapy (e.g. tumor casapase-3 (apoptosis) activation following radiation or chemotherapy). These active biomolecules rarely work in isolation, but are rather parts of integral networks within which any single sub-cellular machine is only transiently active or transiently present, often rapidly giving way to its successor in the signaling chain. This transiency makes these biomolecular machines elusive to analysis by traditional ex vivo methods in the context of the living organism.
However, measuring the activity of these molecules and overcoming the difficulty of target transiency can enable both the detection of disease prior to outward signs and symptoms, and the assessment of the success or failure of therapy prior to disease progression (Aboagye, 2006; Aboagye, 2010). Molecular imaging is a powerful tool for assessing a target biomolecular machine non-invasively and longitudinally over an entire volume of interest in living subjects (James & Gambhir, 2012). The ability to apply molecular imaging to interrogate these highly transient sub-cellular functions of fundamental importance in living subjects is provided by new chemistries for the design of activatable molecular imaging probes over a range of pre-clinical (i.e. fluorescent) to clinically-relevant (PET, MRI) imaging modalities.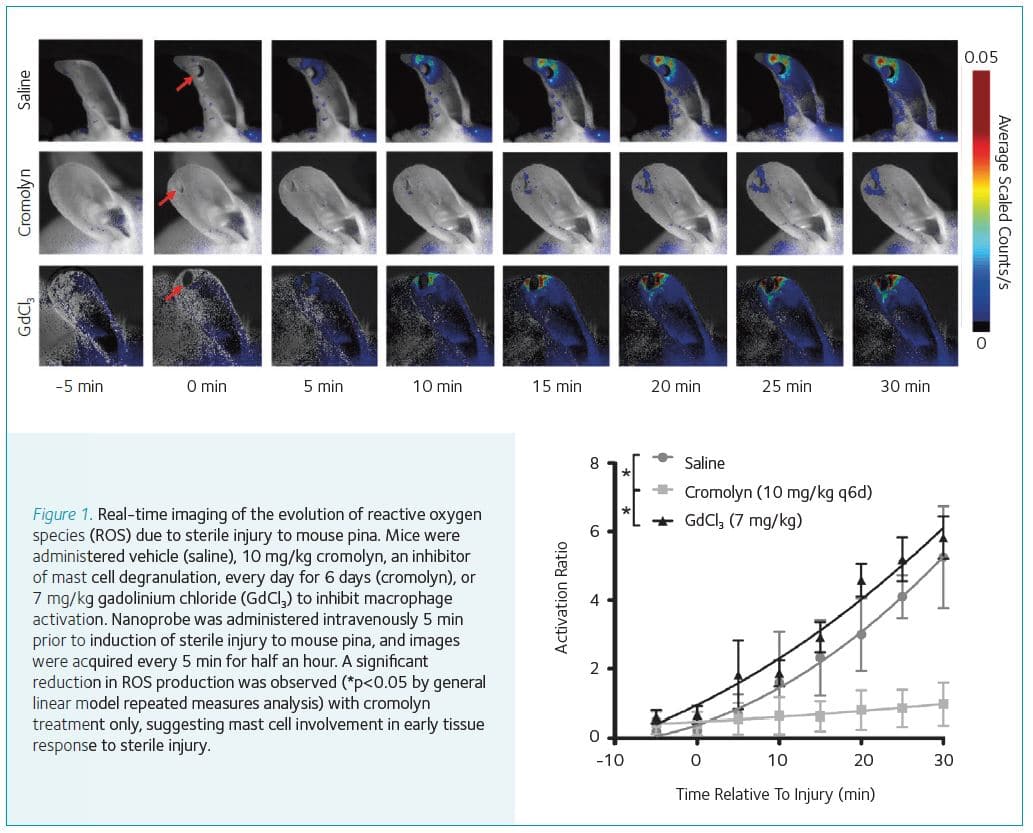
Reactive oxygen species (ROS) are highly reactive oxygen-centered biomolecules underlying a broad range of diseases (Winterbourn, 2008). In the context of injury, ROS are very early effectors of the tissue response to the source of harm. Novel optical nanoprobes have been developed from semiconducting polymers that can sensitively report on the presence of specific ROS in living mice (Pu et al., 2013; Pu et al., 2014; Shuhendler et al., 2014). Our work with the nanoprobes has enabled the real-time detection of the very early response of tissues to mechanical or chemical injury in real-time and in living subjects.
The very early response to tissue injury (i.e. within the first 30 min) have been imaged with evidence pointing to the mast cell component of the innate immune system as being responsible for the very early ROS signaling (Fig. 1; previously unpublished data). Additionally, a dual optical channel probe has been developed capable of the real-time differentiation of hydrogen peroxide versus peroxynitrite mediated drug-induced hepatotoxicity in living subjects (Shuhendler et al., 2014).
‘The molecular imaging of these key sub-cellular machines is sure to continue to provide an unprecedented level of interrogation of physiology to both enhance our investigations of health and disease, and improve clinical outcomes’
Caspase-3 is a cysteine-aspartate protease whose activation signals the committal of the cell to die through apoptosis, a common death pathway induced by a variety of cancer chemotherapeutics and radiation. We designed a modular probe to undergo bioorthogonal macrocyclization upon activation by caspase-3, with the macrocycles self-assembling into nanoparticles in situ in tumor tissue (Shen et al., 2013; Shuhendler et al., 2015; Ye et al., 2014a; Ye et al., 2014b). Since caspase-3 activity correlated with the degree of probe retention in dying tumor tissue, this molecular imaging strategy was applicable to imaging through fluorescence, PET, and MRI. Importantly, this self-assembling molecular imaging probe was able to predict the degree of therapy outcome (i.e. the degree of tumor treatment effect) in mice following a single imaging session 48 hours after a single dose of therapy (Fig. 2).
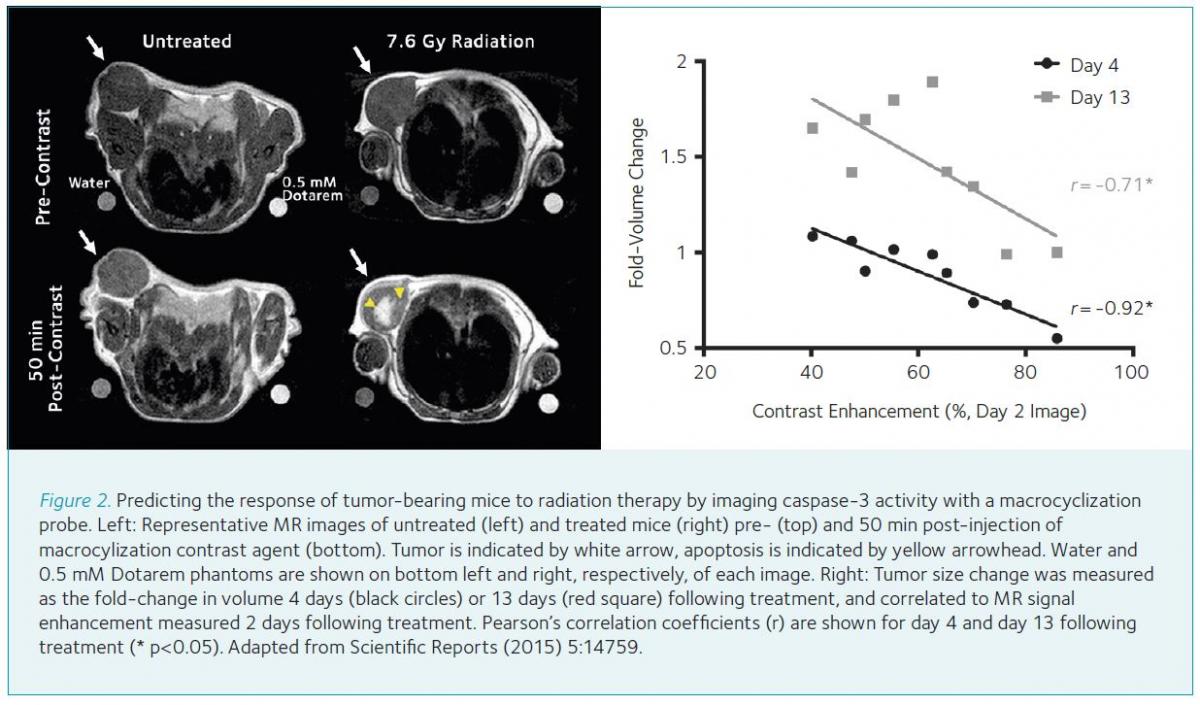
Enhanced contrast production following therapy was not detected using a non-activatable control analog, illustrating the utility of our bioorthogonal self-assembly probe design for therapy response monitoring. Multiple sub-cellular markers for apoptosis have been identified each with molecular imaging probes targeting these sub-cellular changes, including annexin-V detecting phosphatidyl serine flipping from the inner to the outer cell membrane, ML-10 detecting depolarization of the cellular membrane, and fluorodeoxyglucose detecting the loss of metabolic function (i.e. reduced glucose utilization) (Witney et al., 2015). When these targets and respective probes were compared to the macrocyclization probe responding to caspase-3 activation, only the macrocyclizing probe accurately reported tumor response to therapy in living subjects as confirmed by ex vivo measurement of apoptosis (Witney et al., 2015). The ability of the macrocyclization agent to directly probe the effector of apoptotic cell death (i.e. caspase-3) and to significantly amplify the enzyme activity signal by being a substrate of this molecular machine may account for its performance in vivo.
The ability to investigate transient biomolecular machines in living subjects by molecular imaging can provide non-invasive, longitudinal, and predictive data about sub-cellular physiology within the intact organism. The molecular imaging of these key sub-cellular machines is sure to continue to provide an unprecedented level of interrogation of physiology to both enhance our investigations of health and disease, and improve clinical outcomes.
References
Aboagye EO (2006). Imaging in drug development. Clin Adv Hematol Oncol 4, 902–904
Aboagye EO (2010). The future of imaging: developing the tools for monitoring response to therapy in oncology: the 2009 Sir James MacKenzie Davidson Memorial lecture. Br J Radiol 83, 814–822
James ML & Gambhir SS (2012). A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 92, 897–965
Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z & Rao J (2014). Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat Nanotechnol 9, 233–239
Pu K, Shuhendler AJ & Rao J (2013). Semiconducting polymer nanoprobe for in vivo imaging of reactive oxygen and nitrogen species. Angew Chem Int Ed Engl 52, 10325–10329
Shen B, Jeon J, Palner M, Ye D, Shuhendler A, Chin FT & Rao J (2013). Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-triggered nanoaggregation probe. Angew Chem Int Ed Engl 52, 10511–10514
Shuhendler AJ, Pu K, Cui L, Uetrecht JP & Rao J (2014). Real-time imaging of oxidative and nitrosative stress in the liver of live animals for drug-toxicity testing. Nat Biotechnol 32, 373–380
Shuhendler AJ, Ye D, Brewer KD, Bazalova-Carter M, Lee KH, Kempen P, Dane Wittrup K, Graves EE, Rutt B & Rao J (2015). Molecular Magnetic Resonance Imaging of Tumor Response to Therapy. Sci Rep 5, 14759
Winterbourn CC (2008). Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4, 278–286
Witney TH, Hoehne A, Reeves RE, Ilovich O, Namavari M, Shen B, Chin FT, Rao J & Gambhir SS (2015). A Systematic Comparison of 18F-C-SNAT to Established Radiotracer Imaging Agents for the Detection of Tumor Response to Treatment. Clin Cancer Res 21, 3896–3905
Ye D, Shuhendler AJ, Cui L, Tong L, Tee SS, Tikhomirov G, Felsher DW & Rao J (2014a). Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem 6, 519–526
Ye D, Shuhendler AJ, Pandit P, Brewer KD, Tee SS, Cui L, Tikhomirov G, Rutt B & Rao J (2014b). Caspase-responsive smart gadolinium-based contrast agent for magnetic resonance imaging of drug-induced apoptosis. Chem Sci 4, 3845–3852
