Introduction:
Urinary tract infections (UTIs) are one of the most common types of infections in humans. Renal transplant recipients are known to be especially prone to these infections, which can lead to renal damage and graft dysfunction. Normally, the urinary tract is sterile, and antimicrobial peptides (AMPs) play an important role in this as part of the innate immune system. AMPs are generally released into the urine in response to inflammation1. Here, we hypothesise that urine concentration of cathelicidin determines the survival of uropathogenic E. coli in the urinary tract of newly renal transplanted recipients.
Methods:
Urine was collected on day 4 after renal transplantation at Aarhus University Hospital (n=50) and from healthy gender and age-matched controls (n=50). Growth and damage (propidium iodide (PI) assay) of uropathogenic E. coli strain RAR0001 (resistant to sulfamethoxazole and trimethoprim) in the human urine samples was determined by flowcytometry. Urine concentrations of cathelicidin (LL-37), Human b-defensin 1 (HBD-1), lipocalin-2 and uromodulin were determined by ELISA. Direct effect of purified cathelicidin on bacterial damage and lysis was addressed by adding increasing concentrations of cathelicidin measuring damage with a PI-assay and by Green Fluorescence Protein (GFP) release from GFP-producing E. coli.
Results:
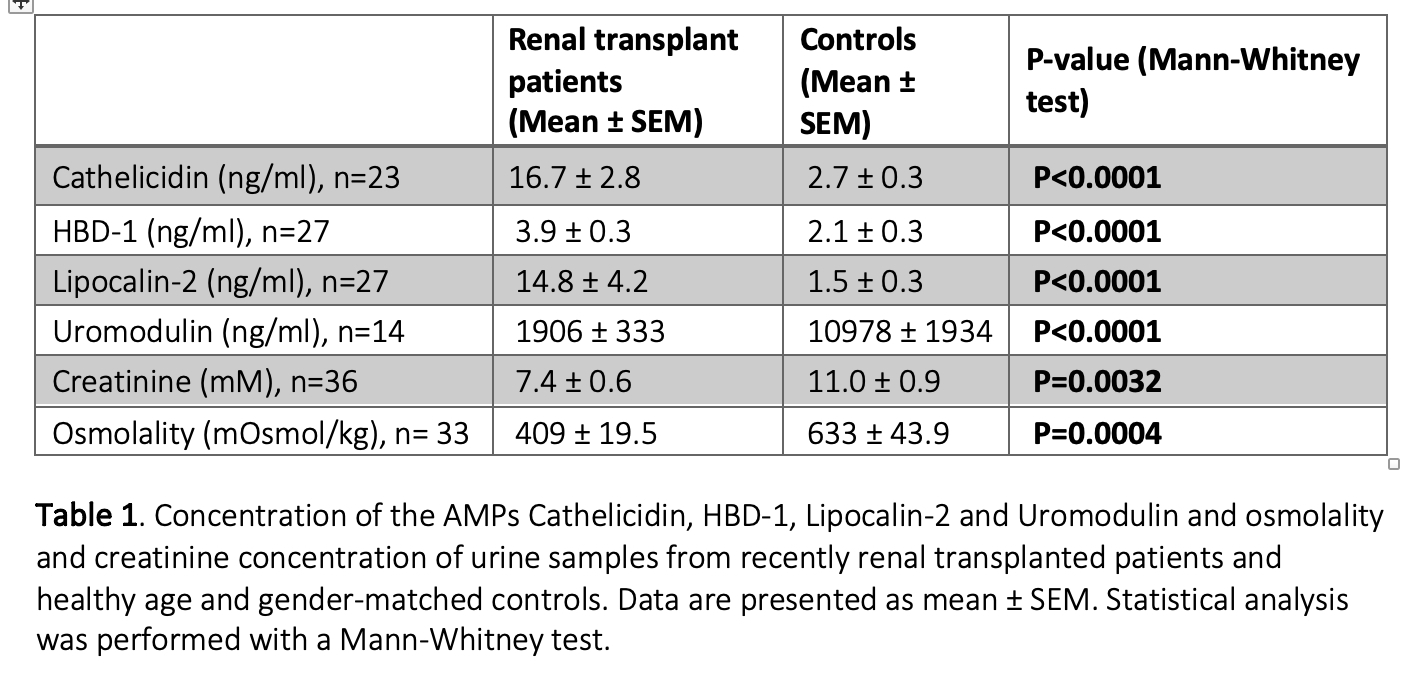
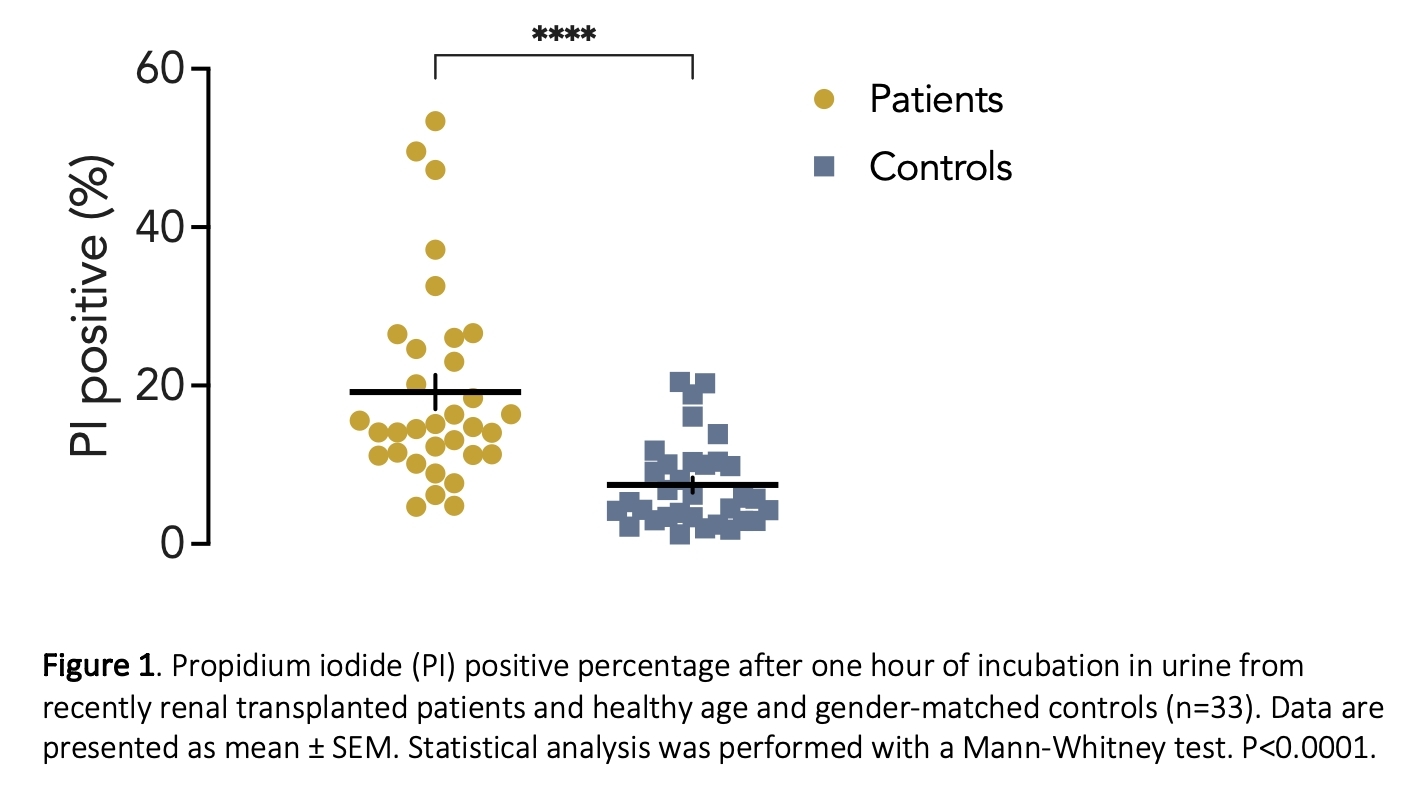
The current study represents preliminary data from 14-36 transplant patients and matched controls, depending on the assay. Urine from renal transplant recipients had a statistically significantly higher mean concentration of the AMPs cathelicidin, HBD-1 and Lipocalin-2 compared to controls (table 1), even though the urine was more dilute as indicated by a lower osmolality and creatinine concentration compared to controls (table 1). Notably, urine uromodulin concentrations were reduced markedly more in renal transplant recipients compared to controls than what could be explained by dilution. Corresponding to the high urinary AMP levels, RAR0001 had a mean of 19.9 ±2.2 % PI-positive bacteria after one hour of incubation in urine from renal transplanted patients (n=33). In contrast, there were only 7.4 ±0.95% PI-positive RAR0001 in urine from controls (n=33) after one hour of incubation (Figure 1, p<0.0001, Mann-Whitney test). Moreover, we confirm that cathelicidin is bactericidal in the used E. coli, since cathelicidin concentration-dependently (0-10 µM) increased the lysis of GFP-producing E. coli (n=3), and cathelicidin (10 µM for four hours) caused 91.5 ±1.2% RAR0001 E. coli to become PI positive (n=3).
Conclusion:
Recently renal transplanted recipients have significantly higher levels of the antimicrobial peptides cathelicidin, HBD-1 and Lipocalin-2, and our in vitro studies confirm bactericidal properties of cathelicidin in uropathogenic E. coli. E. coli is markedly more damaged in urine from these patients compared to urine from healthy controls. We speculate this to be caused by inflammation-induced cathelicidin secretion to the pre-urine caused by the transplantation and propose that higher levels of antimicrobial peptides in the urine can be protective against UTIs in the acute phase after transplantation.