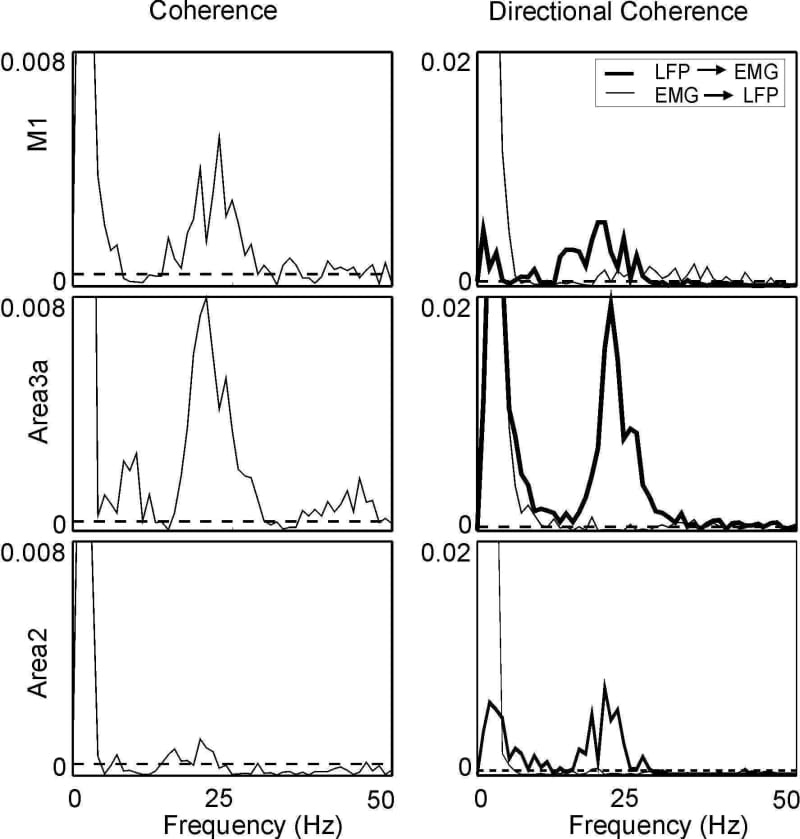
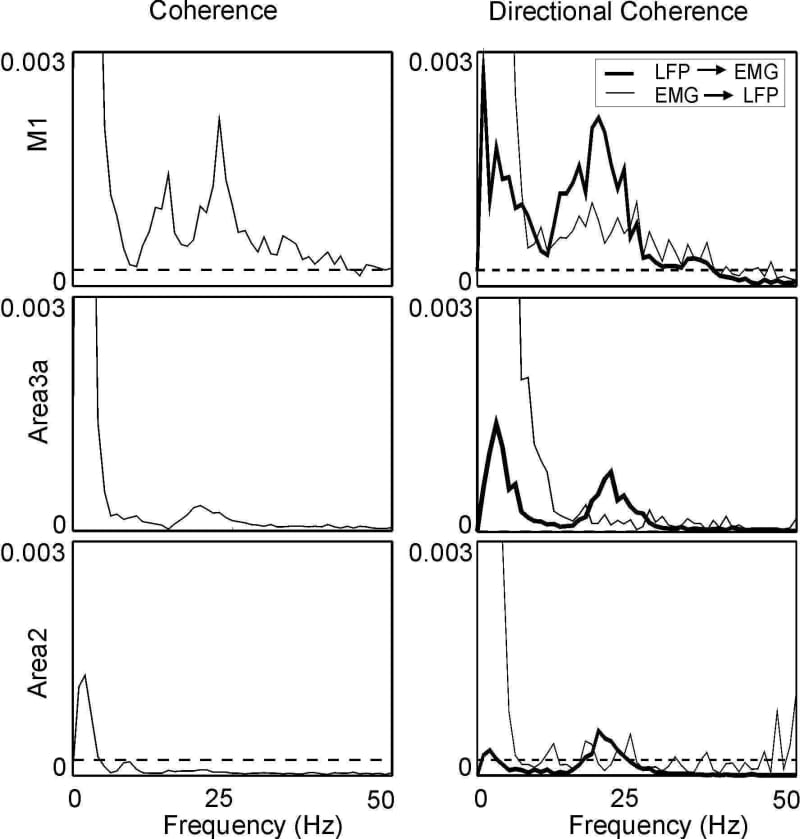
In primates, local field potential (LFP) recordings from primary motor cortex (M1) are coherent with contralateral rectified EMG at 15-35 Hz. Such oscillations may be important for sensorimotor processing; we therefore examined whether somatosensory cortical LFP also shows coherence with EMG. A single macaque monkey was trained to perform a finger flexion task for food reward, and was then implanted (under full general anaesthesia, 3-5% sevoflurane inhalation with 0.025 mg/kg/h alfentanil, and aseptic conditions) with EMG electrodes, a headpiece to allow head fixation, and a recording chamber over the central sulcus. Daily recordings were made from cortical areas 4 (M1), 3a or 2 using glass insulated platinum electrodes or tetrodes. LFP (bandpass 1-100 Hz; referenced to the headpiece) and contralateral EMG from 10 hand and forearm muscles were recorded during task performance. LFP from M1 and area 3a showed significant coherence with EMG in the 20-30 Hz range; coherence with area 2 was weak, and failed to reach significance (P>0.05) when averaged over all 10 available EMG recordings (Figs 1 and 2, left column). Cortico-muscular coherence could be caused by descending pathways from the cortex (e.g. the corticospinal tract), or by ascending inputs carrying sensory feedback from muscle (e.g. the dorsal columns). We investigated these possibilities by calculating directed coherence (DC; Kaminski & Blinowska, 1991), which assesses the extent to which the past history of one signal can predict another. We introduced a novel normalisation for DC: DCij(f)=|Hij(f)|2 Sjj(f)/Sii(f) where Hij(f) is the transfer function, and Sii(f) and Sjj(f) are the power spectra of the two signals. This normalisation makes DC independent of the signal scale. We verified by numerical simulation that the calculation of significance limits (P<0.05; see Evans & Baker, 2003) normally used for standard coherence is also valid for DC with this normalisation. DC in the LFP’EMG direction was significant for all three areas in the 20-30 Hz band. For somatosensory areas, this might be produced by several pathways, including reciprocal cortico-cortical connections with M1. By contrast, although significant DC in the EMG’LFP direction was seen for all areas, it was especially strong for M1 (Figs 1 and 2, right column). We conclude that 20-30 Hz oscillations in areas 3a & 2, as well as M1, can synchronise with muscle activity.
University College London December 2005 (2006) Proc Physiol Soc 1, C12
Oral Communications: Local field potentials from motor and somatosensory cortex show coherence, and directed coherence, with contralateral EMG in the monkey
Wang, Minyan; Witham, Claire L; Baker, Stuart N;
1. School of Clinical Medical Sciences, Sir James Spence Institute, Newcastle Upon Tyne, United Kingdom.
View other abstracts by:
Figure 1. Coherence (left) and DC (right) between LFP and contralateral flexor digitorum profundus (FDP) EMG. Dotted line marks significance level (P<0.05).
Figure 2. Averaged coherence (left) and DC (right) between LFP and 10 contralateral EMGs.
Where applicable, experiments conform with Society ethical requirements.