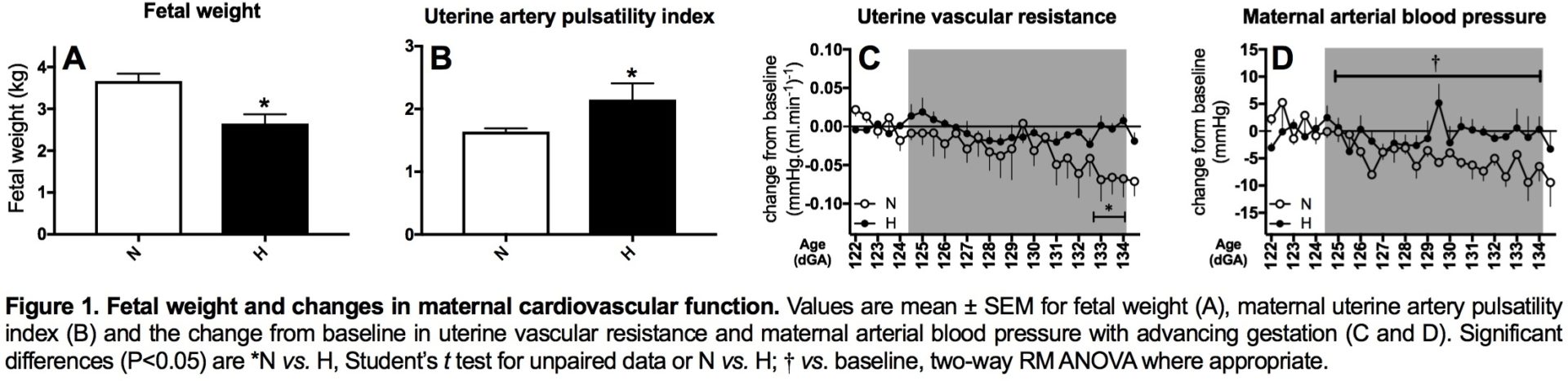
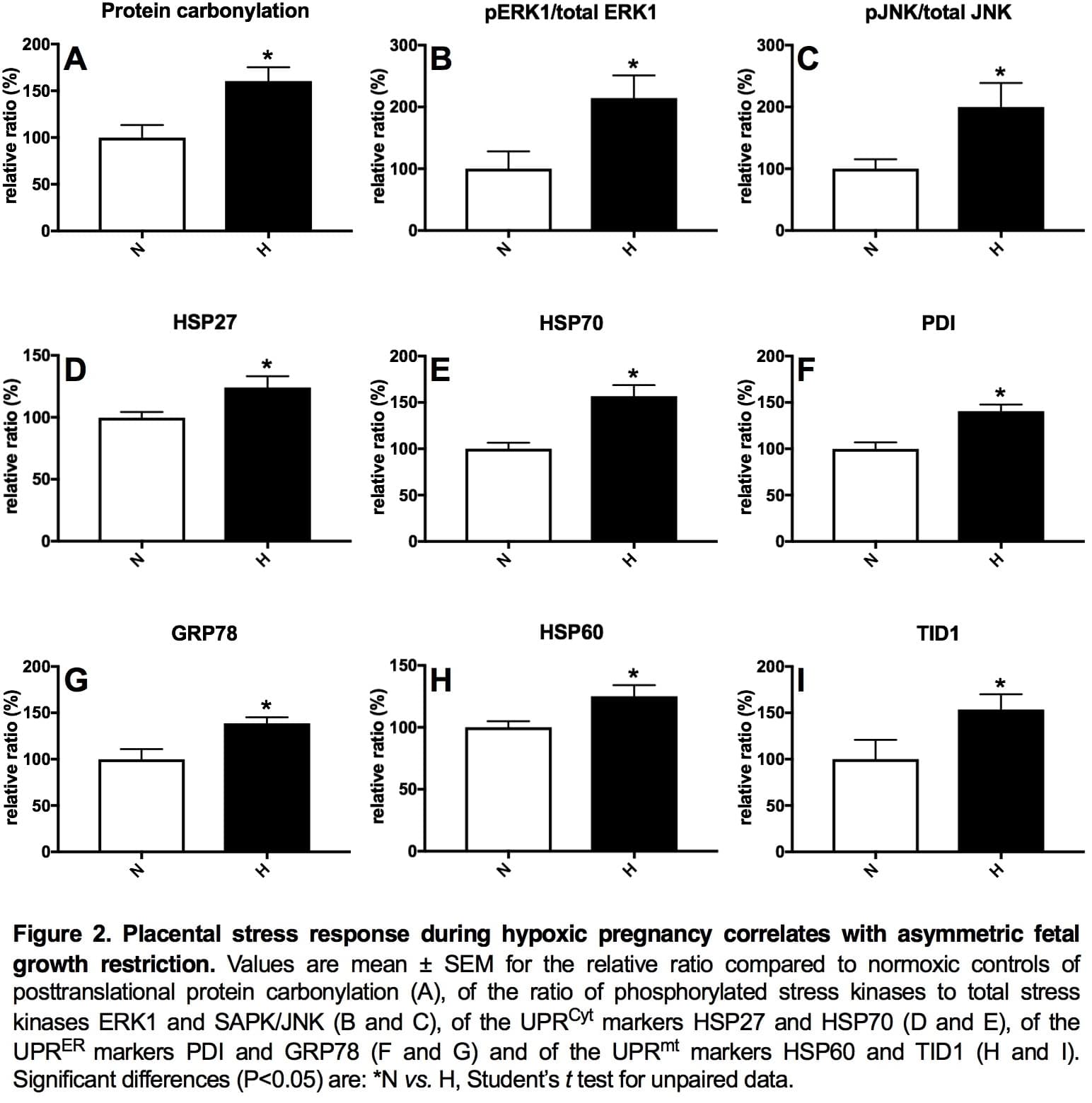
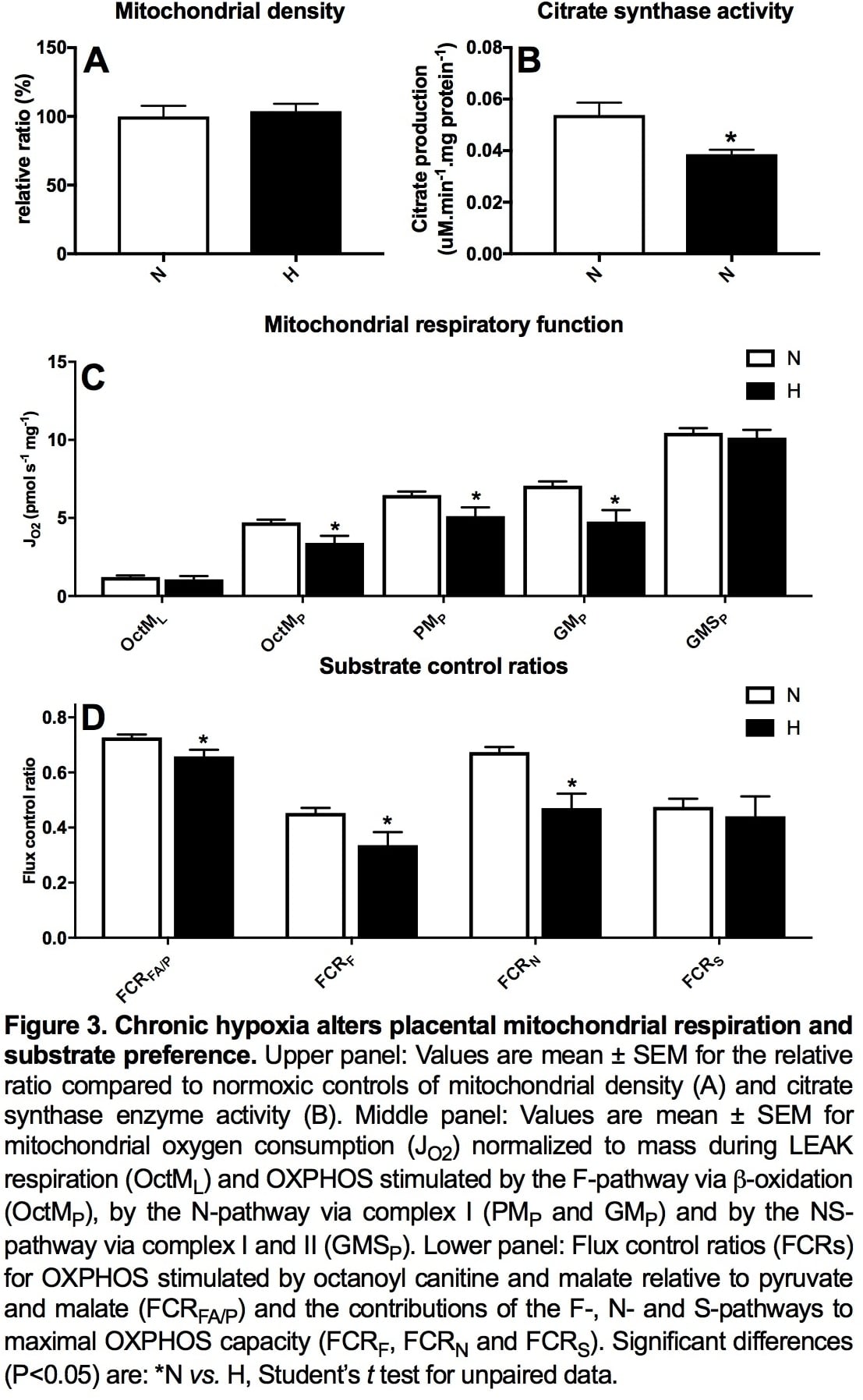
Introduction: Preeclampsia remains a significant killer, affecting the mother and her offspring. However, underlying mechanisms remain uncertain, precluding plausible intervention. We investigated whether hypoxic pregnancy leading to fetal growth restriction (FGR) in sheep results in similar maternal cardiovascular and placental dysfunction, as in preeclampsia. We tested the hypothesis that preeclampsia is linked to hypoxic pregnancy. Methods: Pregnant ewes were exposed to normoxia (N; n=10) or 10% hypoxia (H; n=7) for over 1 month from 105 to 138 days gestational age (dGA; term 145 dGA) in bespoke isobaric chambers. At 138 dGA, in vivo uterine resistance was assessed by Doppler while maintaining N or H, as appropriate. Post mortem, maternal, fetal and placental biometry was determined and placentomes collected for mitochondrial respirometry following permeabilization using saponin and molecular analysis. A separate cohort of pregnant ewes was instrumented at 120 dGA with a uterine Transonic flow probe and vascular catheters to record continuous maternal cardiovascular function via a wireless data acquisition system (CamDAS). Five days later, ewes were exposed to N (n=5) or H (n=5) for 10 days. Data were compared statistically via the Student’s t test, two-way RM ANOVA and the Pearson correlation. Results: Hypoxic pregnancy led to FGR and an increase in uterine artery pulsatility index (PI; Fig 1 A and B). It also prevented the fall in uterine vascular resistance and maternal blood pressure with advancing gestation measured in normoxic controls (Fig 1 C and D). Hypoxic pregnancy was associated with markers of increased placental oxidative stress and of unfolded protein response (UPR) activation in the cytoplasm (UPRCyt), endoplasmic reticulum (UPRER) and mitochondria (UPRmt; Fig 2 A-I). Hypoxic pregnancy did not affect mitochondrial density, but it led to a fall in mitochondrial citrate synthase activity and reduced mitochondrial oxygen consumption dependent on b-oxidation and Complex I (Figure 3). Conclusion: Hypoxic pregnancy leads to placental oxidative and mitochondrial stress associated with alterations in mitochondrial oxygen consumption and energy metabolism. These molecular mechanisms may provide a link between an increase in placental vascular resistance, FGR and maternal cardiovascular dysfunction in adverse pregnancy, as seen in preeclampsia.
Physiology 2019 (Aberdeen, UK) (2019) Proc Physiol Soc 43, C077
Oral Communications: Preeclampsia Link to Hypoxic Pregnancy
W. Tong1,2, K. L. Brain1, B. J. Allison1, K. J. Botting1,2, Y. Niu1,2, S. G. Ford1, P. B. Wooding1, Q. Lyu1, T. A. Garrud1, L. Zhang1, A. Sowton1, K. O'Brien1, T. Cindrova-Davies1,2, B. Yung1,2, G. J. Burton1,2, A. J. Murray1, D. A. Giussani1,2
1. Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge, United Kingdom. 2. Centre for Trophoblast Research, University of Cambridge, Cambridge, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.