Physiology News Magazine
The lymphatic pump – from oedema to envenomation
The lymphatic pump has a key role in tissue fluid homeostasis. Nuclear medicine techniques provide a relatively safe means to investigate lymphatic function, including the pump, in humans. The pump is capable of generating high pressures (as high as diastolic blood pressure), and chronic failure of the pump in lymphoedema of the arm in breast cancer patients may account in part for the swelling
Features
The lymphatic pump – from oedema to envenomation
The lymphatic pump has a key role in tissue fluid homeostasis. Nuclear medicine techniques provide a relatively safe means to investigate lymphatic function, including the pump, in humans. The pump is capable of generating high pressures (as high as diastolic blood pressure), and chronic failure of the pump in lymphoedema of the arm in breast cancer patients may account in part for the swelling
Features
Anthony Stanton
Cardiac & Vascular Sciences, St George’s, University of London, UK
https://doi.org/10.36866/pn.72.15
Oedema develops when capillary filtration exceeds the capacity of the lymphatic drainage system for a substantial period. Oedema is a familiar medical condition, whether it be the dependent oedema of the feet, the angioedema of allergy, the pulmonary or systemic oedema of cardiac failure (hyperfiltration oedemas), or the chronic (lymph) oedema occasioned by lymphatic insufficiency. What do we know of the lymphatic side of things?Starling, writing on the movement of lymph in his short textbook of human physiology in 1892 (and doubtless drawing on Heller’s work some 20 years previously) referred to lymphatic vessels as channels with rhythmically contracting muscular sacs and valves that pump lymph (Starling, 1892). He discussed the significance of pressure gradients, active and passive limb movements, and noted the low to zero flow in the resting limb.
Cannulating lymphatics
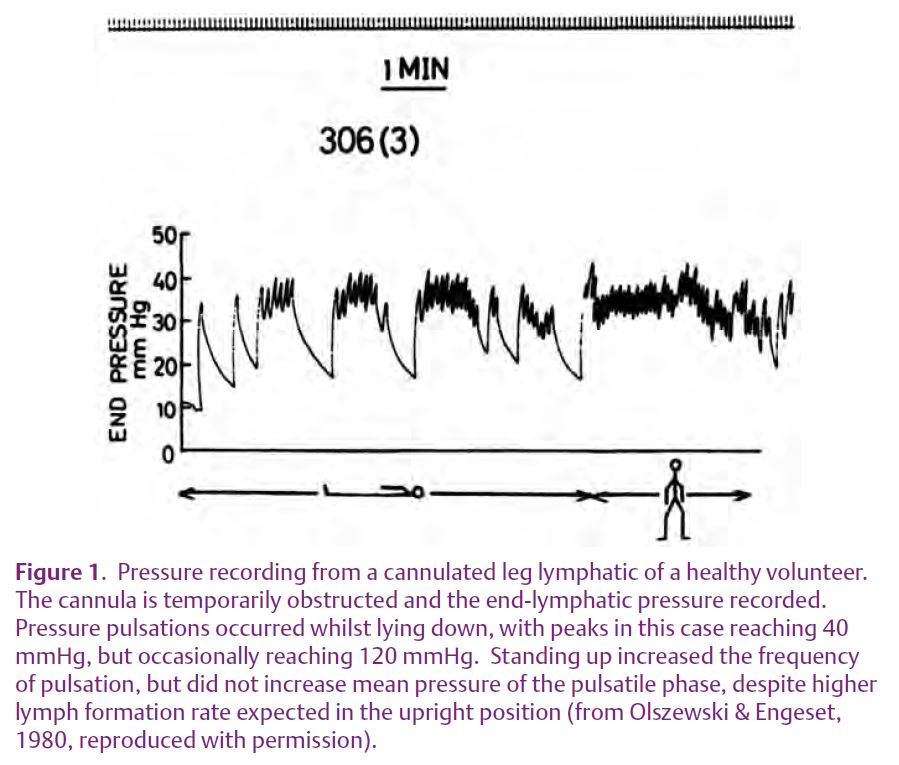
Moving on almost a century, Olszewski & Engeset (1980) recognised that peripheral lymph propulsion was poorly characterised and addressed the question ‘How is lymph propelled along the peripheral lymphatics of humans?’ Subcutan-eous lymphatic vessels draining the foot and anterior lower leg were cannulated in healthy men and, with the cannula left in situ, lymph flow and pressure were measured a few days later. With the subject supine and the cannula connected to a pressure transducer with flow obstructed, pressure pulsations were recorded with peaks usually in the range 12–55 mmHg, but occasionally much higher (120 mmHg). Standing up increased pulse frequency (Fig. 1). With free flow of lymph, peak pressures were lower (7–30 mmHg) and flow averaged 0.25 ml h-1, rising 6-fold during a pulse wave. Foot and leg movements did not increase the peak pressures but did increase the frequency of pulsation and flow, which appeared to be linked. Lymph flow occurred only during the pressure pulsations. This excellent study thus established that human prenodal lymphatic collector vessels in the leg contract rhythmically to propel lymph centrally. Interestingly, by artificially raising the intraluminal pressure, spontaneous contractions were evoked. The authors also thought that extrinsic skeletal muscle contractions facilitated flow by increasing lymph formation (capillary filtration and lymph flow are closely coupled) and by an external pumping action on the non-contractile initial lymphatics.
Simulating envenomation
The above approach, involving surgical cutdown, was invasive and cannot be used ethically to study patients. Over the past 20 years or so less invasive techniques have been developed, based on gamma camera imaging of lymphatic transport. Howarth et al. (1994) used this approach to see what tourniquet pressure is needed to halt lymph drainage during the first-aid treatment of snake or spider envenomation. They simulated envenomation by injecting a radiotracer (99mTc-antimony sulphur colloid) intradermally or subcutaneously in the ankle or wrist and measured the transit time to the regional lymph nodes and the systemic circulation. Pressure was applied to the limbs by bandaging proximally from the depot, initially with the limb immobilised and the subject supine. Bandage pressures of 55–70 mmHg held for 30–145 min prevented radiotracer transit from ankle to inguinal nodes in most subjects; pressures < 55 mmHg did not prevent transit and a pressure of 90 mmHg for 35 min did not prevent transit in one subject. In the arm, pressures as high as 85–110 mmHg applied for 25–45 min (and causing numbness) failed to prevent transit from wrist to axilla in 6 of the 15 subjects; 40–70 mmHg was effective in just under half the subjects. Exercise promoted transit. The findings of Howarth et al. thus reinforce the findings of Olszewski & Engeset (1980) that human limb lymphatics are capable of powerful contractile activity.
Probing the pump in lymphoedema
In recent years, exploration of lymphatic function in humans using nuclear medicine techniques has focused on the arms of women treated for breast cancer. Damage to the lymphatic drainage paths running through the axilla by the surgery or radiotherapy often results in a delayed, disfiguring arm swelling called lymphoedema. Using a development of Howarth’s approach, Modi et al. (2007) assessed lymphatic contractility in healthy control arms and in the lymphoedematous arms of women treated for breast cancer. Their technique, called lymphatic congestion lymphoscintigraphy (LCP), employed a mercury sphygmomanometer and Riva-Rocci cuff to apply pressure around the upper arm. By injecting radiotracer (99mTc-IgG) intradermally into the hand of the supine subjects and gradually reducing the cuff pressure whilst imaging the upper arm and axilla, the pressure that the forearm lymphatic collectors could overcome (the ‘pump pressure’) could be established (Fig. 2). The aim was to explore the idea that, following surgical excision of axillary lymph nodes, lymphoedema occurred because of impaired intrinsic contractility of the superficial collector lymphatics in the face of chronically increased resistance to flow downstream.
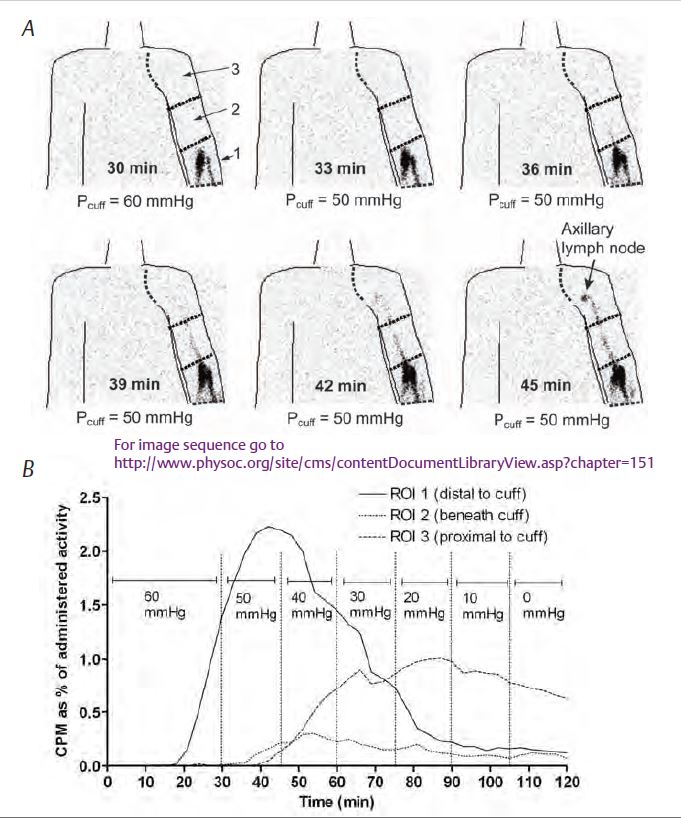

In the absence of cuff compression, transit from the hand to the axilla was found to be remarkably fast, < 3 min in one subject and the longest time was 21 min. With the cuff in place in healthy individuals, the radiotracer flowed up the forearm and under the cuff to reach the axilla at an average cuff pressure of 39 mmHg, but at higher pressures the radiotracer could not get past the cuff. Thus, the mean lymphatic collector pump pressure was 39 mmHg, though with a wide range (10–60 mmHg). Blood accumulation of tracer increased sharply after the tracer reached the axilla, as the radiolabelled lymph progressed to the thoracic duct and entered the great veins, with a further surge in blood counts when the subject later stood up and moved around.
In lymphoedematous arms the pump pressure was significantly impaired, averaging 24 mmHg. An image sequence showing the progression of activity up the arm in one patient is available at the above address.
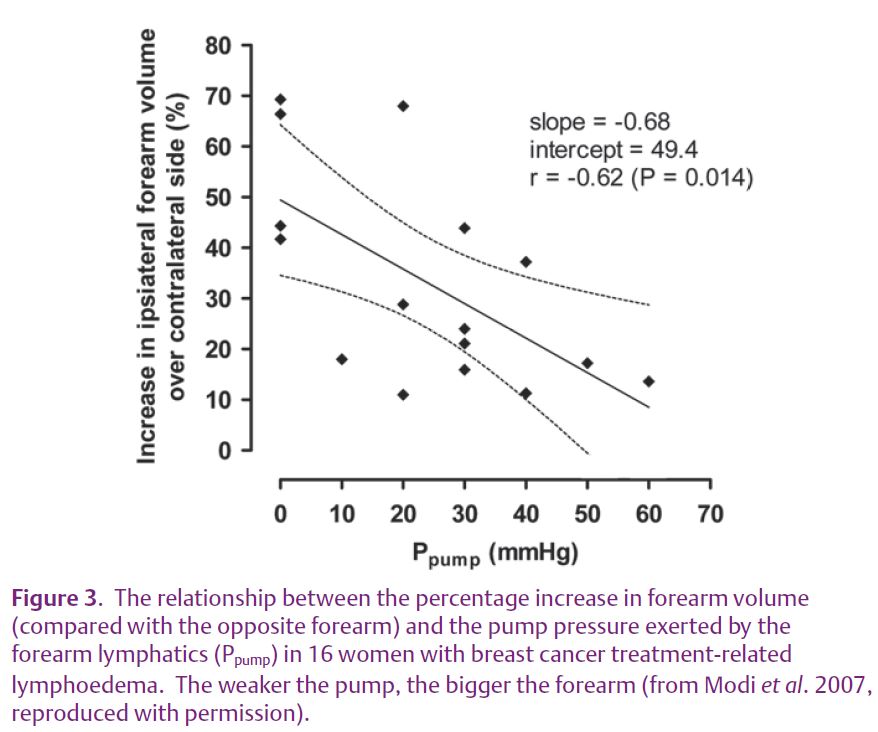
The range of pump pressures was again wide (0–60 mmHg) and the pump pressure correlated with the degree of swelling (Fig. 3). The overlap of pump pressures with the healthy arms may indicate that additional factors besides impaired pump contractility contribute to the lymphoedema. The blood surge after the axilla was reached was less marked, in keeping with impaired lymph flow in the swollen arm, but the surge on standing still occurred.
A hypothesis
The authors noted the similarity of this concept of lymphatic failure with hypertensive cardiac failure. They proposed that a chronically increased afterload causes the lymphatic collector pump to fail after some months, resulting in the characteristically delayed onset of post-breast cancer treatment lymphoedema. But why do some women develop lymphoedema after the cancer treatment and some not? Possible explanations include constitutionally weaker lymphatic pumping in some individuals and/or a higher pre-morbid peripheral lymph flow, for which there is preliminary, unpublished evidence. These possibilities have implications for women recently diagnosed with breast and other forms of cancer and could be explored using recently developed, almost non-invasive nuclear medicine techniques. These techniques would also offer a way of assessing putative pharmacological therapies for lymphatic failure.
Conclusions and the future (and what to do after a bite)
The wide range of lymphatic function in health and disease was a notable feature of all the above studies. Rapid lymphatic transport (Modi et al. 2007) was also noted by Aas et al. (1985) who injected radiotracer subcutaneously in the foot of sitting subjects and detected activity in the thigh in < 1 min and in the systemic blood in 2–5 min. This makes evident the urgency of commencing first-aid measures following snake or spider bites, which are aimed at collapsing the superficial lymphatics (and veins) by compression and immobilisation, or by placing a constricting band around the limb, so as to reduce flow. Even high external pressures may fail to arrest lymphatic transport, and such pressures would in any event have to be guessed at in the field. There is also the possibility of transport of toxin from the superficial (epifascial) to the deep (subfascial) lymphatic systems, which are connected in the limbs and trunk.
The best advice is, clearly, don’t get bitten!
Our understanding of the lymphatic pump, and how it is primed, is still lacking, although its importance in tissue homeostasis is recognised (Levick & McHale, 2003; Mortimer & Levick, 2004). The high prevalence of cancer treatment-related lymph-oedema may serve as an impetus for future work; lymphoedema is a problem that will not go away and may even become commoner as more patients survive cancer. Curiously, breast cancer-related lymphoedema still occurs in at least 6% of patients after sentinel lymph node biopsy, in which only 1–2 lymph nodes are removed (the incidence after standard axillary treatment is perhaps 1-in-4), whereas sometimes it does not occur after complete axillary clearance surgery. A number of current clues point to a genetic predisposition to this form of lymphoedema and this may prove an exciting avenue for future exploration, with the potential to identify and treat high risk patients.
Acknowledgement
With thanks to Rodney Levick and Peter Mortimer for their support.
References
- Aas M, Skretting A, Engeset A, Westgaard R & Nicolaysen G (1985). Lymphatic drainage from subcutaneous tissue in the foot and leg in the sitting human. Acta Physiol Scand 125, 505–511.
- Howarth DM, Southee AE & Whyte IM (1994). Lymphatic flow rates and first-aid in simulated peripheral snake or spider envenomation. Med J Aust 161, 695–700.
- Levick JR & McHale N (2003). The physiology of lymph production and propulsion. In: Diseases of the lymphatics, pp 44–64. Arnold, London.
- Modi S, Stanton AWB, Svensson WE, Peters AM, Mortimer PS & Levick JR (2007). Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol 583, 271–285.
- Mortimer PS & Levick JR (2004). Chronic peripheral oedema: the critical role of the lymphatic system. Clin Med 4, 448–453.
- Olszewski WL, & Engeset A (1980). Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol 239, H775–H783.
- Starling EH (1892). Elements of human physiology. J & A Churchill, London.