
Physiology News Magazine
Oxygen and the ocular lens
A key to understanding nuclear cataract?
Features
Oxygen and the ocular lens
A key to understanding nuclear cataract?
Features
Richard McNulty (1,2) & Steven Bassnett (1)
1: Department of Ophthalmology, Washington University, St Louis, Missouri, USA
2: Australian Cataract Research Foundation, Wollongong University, NSW, Australia
https://doi.org/10.36866/pn.60.18

The ocular lens is located behind the iris and in front of the gel-like vitreous humour. Normally the lens cannot be seen, and with good reason: its function is to transmit and focus light. Any disturbance in lens metabolism is likely to result in opacification of the tissue, a condition known as cataract. Although eating a diet rich in anti-oxidants and vitamins may slightly decrease one’s chances of getting a cataract, the only successful treatment currently available is surgical removal of the cataractous lens.
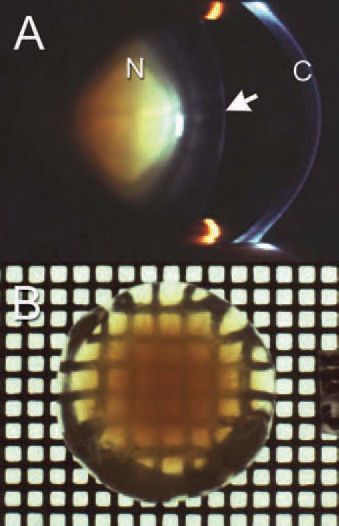
The most common type of cataract is age-related nuclear cataract (ARNC), a yellowy-brown cataract located in the lens centre (Fig. 1). This type of cataract is probably the single leading cause of global blindness. The concept that molecular oxygen may play a key role in the development of ARNC has emerged from two types of study.
Firstly, detailed biochemical analyses of ARNC cataracts have revealed widespread oxidation of lens proteins. Secondly, oxygen has been implicated directly in the cataracts that form as an unwanted side effect of certain clinical procedures. For example, nuclear cataracts often appear in patients following hyperbaric oxygen (HBO) therapy or vitrectomy surgery (Palmquist et al. 1984; Holekamp et al. 2005). HBO appears to overwhelm the lens with oxygen, causing oxidative stress. During vitrectomy, a surgical treatment for retinal disease, the vitreous gel inside the eye is removed and replaced with a physiological salt solution. Convective mixing of this solution within the eye may lead to the delivery of oxygen from the highly vascular retina to the lens. Because HBO- and vitrectomy-associated cataracts closely resemble ARNC clinically and biochemically, it has been postulated that oxygen may also have a causative role in the latter.
The lens is normally shielded from the potentially damaging effects of oxygen by virtue of both its location and unusual physiology. The interior of the eye is a relatively hypoxic environment, containing levels of oxygen only 10% of that found in arterial blood. The lens lacks a blood supply (hemoglobin would absorb light) and oxygen destined for the inner cells must, therefore, diffuse through the superficial tissue layers. Recently, we proposed a new model of the lens in which metabolic processes in these outer cell layers generate standing gradients of oxygen within the tissue. As a result, cells of the lens core exist in a permanently hypoxic state (Fig. 2).

Only the outermost, and youngest, lens cells have a full complement of organelles. Respirometric measurements have indicated that mitochondria located in these cells consume 90% of the oxygen entering the lens and, by so doing, reduce the concentration of dissolved oxygen in the lens core to <2 µM (McNulty et al. 2004). In these permanently hypoxic cells the introduction of oxygen is likely to have profound and deleterious effects. Interestingly, the lens meets most of its energy needs not from oxidative phosphorylation but from glycolysis. Indeed, various indices of lens viability (including tissue clarity) are preserved in the complete absence of oxygen. Perhaps uniquely in this tissue, therefore, the role of mitochondria in generating ATP via oxidative phosphorylation might be secondary to their role in excluding oxygen from the lens centre.
Because lens core hypoxia appears to depend critically on mitochondrial function, manipulations that interfere with mitochondrial metabolism result in the rapid flooding of the core with oxygen. For example, simply lowering the temperature from 37oC to room temperature is sufficient to cause a rapid rise in oxygen concentration in the lens core. This may be a clinically pertinent observation because chilled solutions are often infused into the eye during ocular surgery. As noted above, nuclear cataract is an unfortunate side effect of many such surgeries and may result, at least in part, from the exposure of the lens core to oxygen during the procedure. With aging, mitochondrial function in general is known to decline. It remains to be determined whether reduced mitochondrial oxygen consumption in the outer cell layers of the aging lens compromises core hypoxia and thus triggers nuclear opacification.
Acknowledgement
These studies were supported by NIH grants R01-EY015507 (to SB), EY02687 (Core Grant for Vision Research) and an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. SB is an RPB William and Mary Greve Scholar.
References
Holekamp NM, Shui YB & Beebe DC (2005). Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol 139, 302-310.
Marcantonio JM, Duncan G, Davies PD, Bushell AR (1980). Classification of human senile cataracts by nuclear colour and sodium content. Exp Eye Res 31, 227-237.
McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ & Bassnett S (2004). Regulation of tissue oxygen levels in the mammalian lens. J Physiol 559, 883-898.
Palmquist BM, Philipson B & Barr PO (1984). Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol 68, 113117.
