
Physiology News Magazine
Pharyngeal airway mechanics during muscle stimulation by tagged magnetic resonance imaging
A novel application of MRI tissue tagging was used to track tissue motion in the pharyngeal walls due to contraction of tongue protrudor muscles that dilate the airway. Airway enlargement in the antero-posterior and lateral dimensions was associated with ventrally directed displacement of the lateral and ventral pharyngeal walls. The results reveal complex relationships between changes in airway size and pharyngeal wall tissue motion
Features
Pharyngeal airway mechanics during muscle stimulation by tagged magnetic resonance imaging
A novel application of MRI tissue tagging was used to track tissue motion in the pharyngeal walls due to contraction of tongue protrudor muscles that dilate the airway. Airway enlargement in the antero-posterior and lateral dimensions was associated with ventrally directed displacement of the lateral and ventral pharyngeal walls. The results reveal complex relationships between changes in airway size and pharyngeal wall tissue motion
Features
Michael J Brennick (1)
Samuel T Kuna (2)
1: Center for Sleep and Respiratory Neurobiology, University of Pennsylvania, Philadelphia, USA
2: Department of Medicine, University of Pennsylvania and Pulmonary, Critical Care and Sleep Section, Philadelphia Veterans Administration Medical Center, Philadelphia, USA
https://doi.org/10.36866/pn.59.32
Obstructive sleep apnoea (OSA) is a respiratory disorder in humans characterized by the repetitive closure of the pharyngeal airway during sleep. It is widely acknowledged that both anatomical and physiological factors may predispose an individual to OSA. Schwab et al. (2003) have used magnetic resonance imaging (MRI) to show that patients with OSA have smaller pharyngeal airways and thicker lateral pharyngeal walls than individuals without OSA. While standard MRI techniques can determine airway dimensions and the geometry of pharyngeal wall soft tissue structures, they cannot directly examine pharyngeal mechanics, i.e. pharyngeal wall tissue motion.
Physiological factors include the function of skeletal muscles in the pharynx that dilate and stiffen the airway. Selective neural stimulation of pharyngeal dilating muscles during sleep has been studied as a possible treatment to prevent airway closure (Schwartz et al. 1996). Although previous studies in animals and humans have examined the increase in airway size and stiffness due to pharyngeal muscle stimulation, there is little information as to how activation of the pharyngeal muscles alters the soft tissues in the pharyngeal walls to effect these airway changes (Fuller et al. 1999; Kuna & Brennick, 2002).
To address these limitations, we have adopted a novel approach using MRI with spatial modulation of magnetization (SPAMM®) (Axel & Dougherty, 1989) to track tissue motion in the pharyngeal wall during muscle stimulation (Brennick et al. 2004). We examined how stimulation of the medial branch of the hypoglossus nerve, supplying motor output to protrudor (genioglossus and geniohyoid) and intrinsic tongue muscles, affects the displacement and strain of tissues surrounding the pharyngeal airway and how the pharyngeal wall tissue motion relates to changes in airway size and shape. Eleven Sprague-Dawley rats were surgically prepared with platinum electrodes for bilateral stimulation of the medial branch of the hypoglossus nerve, and images of the pharyngeal airway were acquired before and during stimulation using a spoiled gradient recalled imaging (SPGR) MRI protocol in a 4.7T magnet. Image analysis used customized software including optical flow displacement and finite element analysis.
Airway dimensions measured before and during stimulation showed that cross-sectional area, and anteroposterior and lateral dimensions in the oropharyngeal and nasopharyngeal airways, were significantly increased when results were averaged across the rostral, mid- and caudal pharynx (p values <0.001).
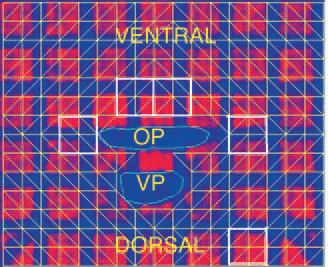
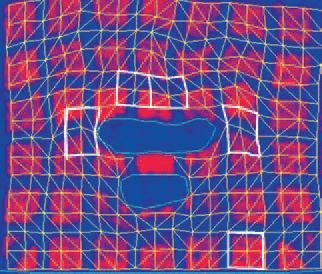
Fig. 1 shows images of a single axial slice in the caudal pharynx before (top image) and during stimulation (lower image). Note the change from the perpendicular grid pattern prior to nerve stimulation (top panel) to the distorted pattern (lower panel) during stimulation. Pharyngeal wall tissue displacement and strain were determined bilaterally in tissue sectors in the lateral and ventral pharyngeal walls surrounding the oropharyngeal airway and compared with a control sector in the brain.
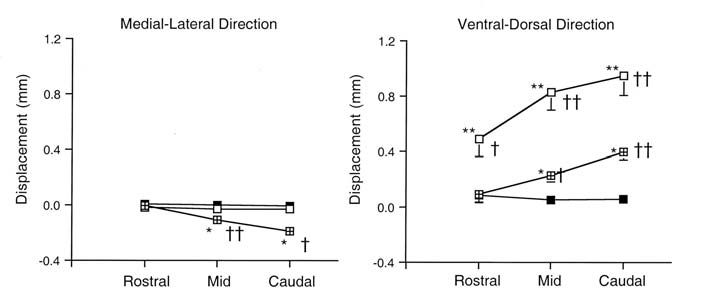
Stimulation caused ventral displacement of tissues in the ventral pharyngeal walls in all regions (p<0.0032) and ventral displacement of the lateral walls in the mid- and caudal regions (p<0.0001) (Fig. 2). In addition, in the mid- and caudal regions, stretch (principal maximum strain) of lateral sectors was significantly greater than that of control sectors (p<0.023). As there was no lateral displacement of the lateral pharyngeal walls at any rostral to caudal pharyngeal level examined, these results suggest that airway dilation during stimulation of the medial branch of the hypoglossal nerve was predominantly due to ventral displacement of the ventral and lateral pharyngeal walls.
We believe this novel MRI application will lead to a better understanding of airway phenomena such as how dilator muscles act to maintain pharyngeal patency and how airway collapse occurs in different regions of the pharynx.
Acknowledgement
Funding: NIH HL-27520 and NIH EB-01780.
References
Axel L & Dougherty L (1989). MR imaging of motion with spatial modulation of magnetization. Radiology 171, 841-845.
Brennick MJ, Pickup S, Dougherty L & Kuna ST (2004). Pharyngeal airway wall mechanics using tagged magnetic resonance imaging during medial hypoglossal nerve stimulation in rats. J Physiol 561, 597-610.
Fuller DD, Williams PL, Janssen PL & Fregosi RF (1999). Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519, 601-613.
Kuna ST & Brennick MJ (2002). Effects of pharyngeal muscle activation on pressure-area relationships. Am J Respir Crit Care Med 166, 972-977.
Schwab RJ, Pairstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G & Pack AI (2003). Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 168, 522530.
Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D & Smith PL (1996). Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol 81, 643-652.
