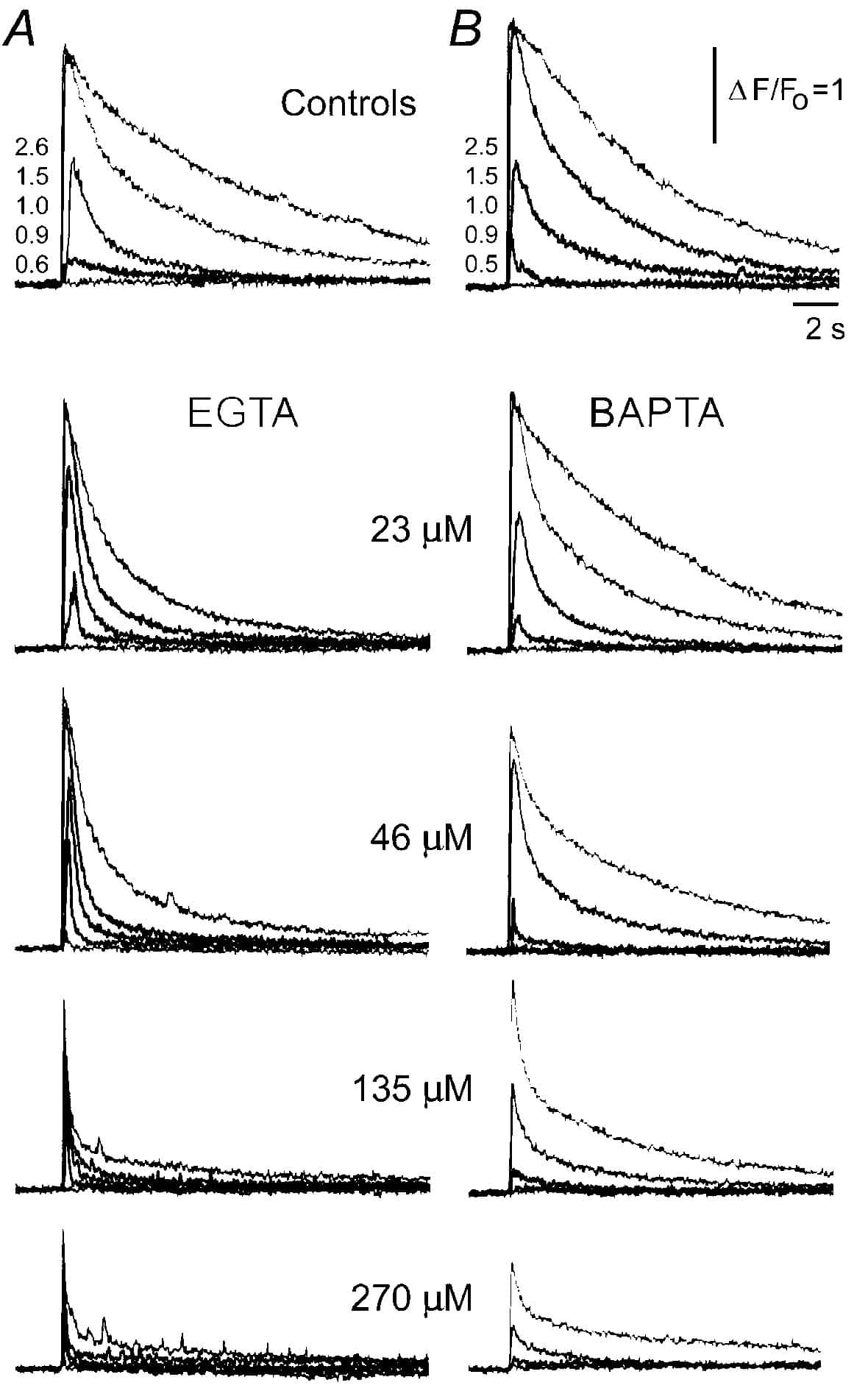
Cellular calcium buffers shape cytosolic calcium signals by regulating the availability and diffusional mobility of free calcium ions. Most experimental and theoretical studies of these effects have concentrated on signals arising from a fixed ‘pulse’ of calcium – for example, calcium entry through voltage-gated channels. A more complex case involves intracellular calcium liberation through inositol trisphosphate (IP3) receptors, which are themselves regulated by calcium. Thus, cytosolic buffers are expected to influence the successive cycles of diffusion and calcium-induced calcium release that act both between within clusters of IP3 receptors (to generate local ‘puffs’), and adjacent clusters (to generate global calcium waves).
We studied such interactions in Xenopus oocytes, using confocal line-scan microscopy together with photo-release of IP3. Intracellular injections of buffers with ‘slow’ calcium-binding kinetics (EGTA and parvalbumin) speeded the decay of calcium signals (Fig. 1A) and ‘balkanized’ IP3-evoked calcium waves into discrete puffs. Contrastingly, equivalent concentrations ‘fast’ buffers (BAPTA and calretinin) slowed IP3-evoked calcium responses (Fig. 1B) and promoted ‘globalization’ of spatially uniform calcium signals. These observations likely reflect buffer actions on calcium feedback at IP3 receptors, since the effects of EGTA and BAPTA on calcium signals evoked by influx through expressed N-type voltage gated channels were markedly less pronounced. Moreover, they point to the importance of buffer kinetics in shaping IP3-evoked calcium signals; likely because fast buffers can influence calcium feedback between individual IP3R within a cluster, whereas slow buffers are able to modulate only cluster-cluster interactions. We propose that cell-specific expression of calcium binding proteins with distinct kinetics may shape the time course and spatial distribution of IP3-evoked calcium signals for specific physiological roles.
This work was supported by NIH GM 48071 and GM 58329