Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterised by both motor and non-motor symptoms, significantly impacting quality of life. Deep brain stimulation (DBS) has emerged as an established therapeutic intervention for advanced PD, particularly in patients with motor complications refractory to pharmacological treatment. However, heterogeneity in clinical presentation across PD subtypes, such as tremor-dominant (TD), postural instability and gait difficulty (PIGD), akinetic-rigid (AR), and mixed (MX), suggests that outcomes may not be uniform. The majority of studies confirmed the efficacy of DBS in a general way, but they do not shed much light on which subtype gains the most, nor on which symptoms should continue to defy treatment (Eghlidos et al., 2022). This rapid review evaluated outcomes of deep brain stimulation in Parkinson’s disease, comparing motor, non-motor, and quality-of-life outcomes across established motor subtypes. In addition, this review offers a specific analysis of outcomes by motor subtypes and by item scores derived from the UPDRS.
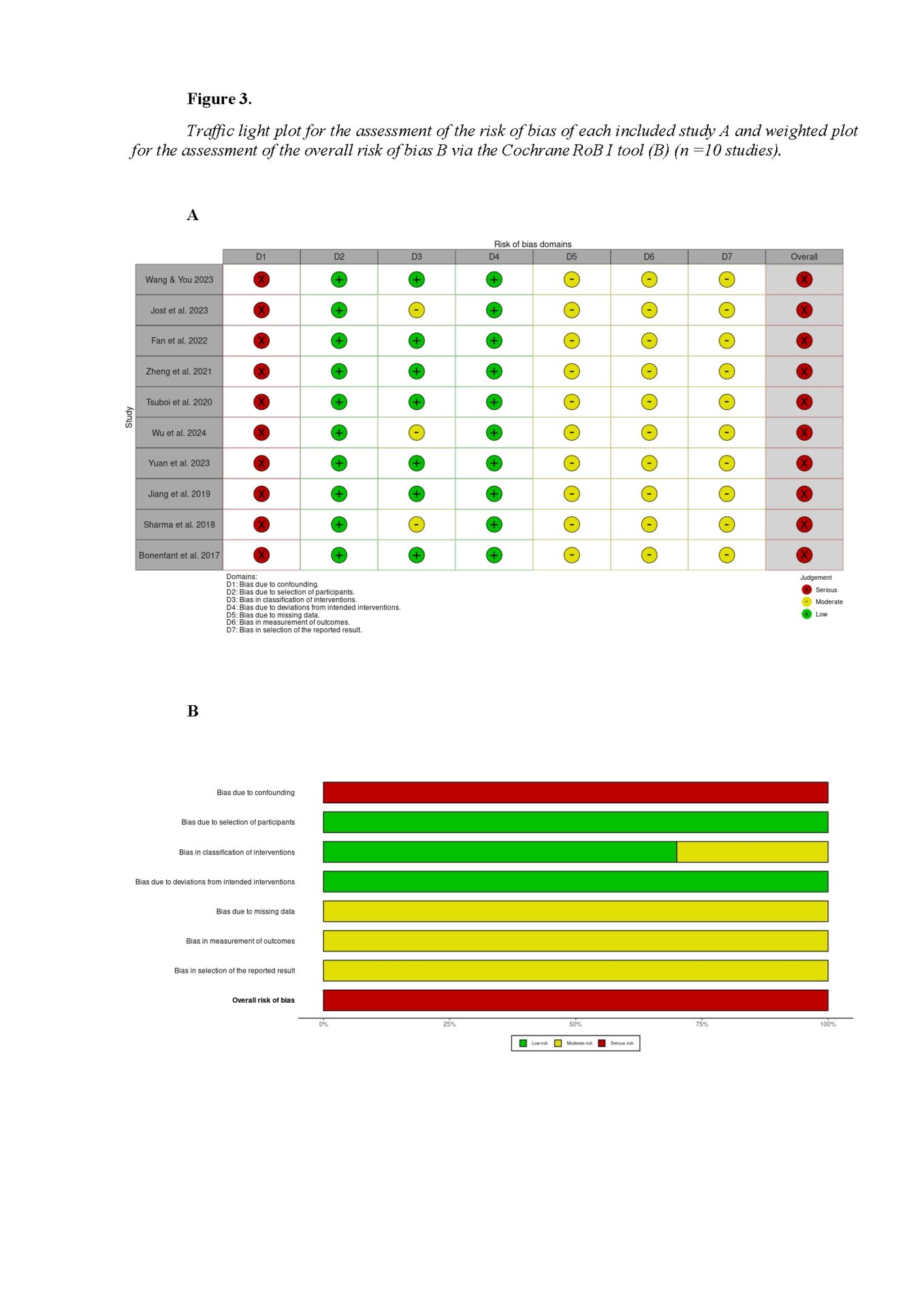
A structured review protocol guided the study to ensure transparency and best practices. The review followed PRISMA 2020 guidelines (Page et al., 2021). Researchers searched PubMed, Scopus, Web of Science, and Embase. Eligibility criteria followed the PICO model and informed inclusion and exclusion rules. Risk of bias was assessed in all ten studies using the ROBINS-I tool. A systematic search found ten eligible studies; five reported outcomes by subtype and were narratively synthesized. The other five did not stratify by subtype, but gave UPDRS item subscores for tremor (items 20–21), rigidity (item 22), bradykinesia (items 23–26, 31), and axial symptoms (items 27–30); these formed the basis for meta-analysis.
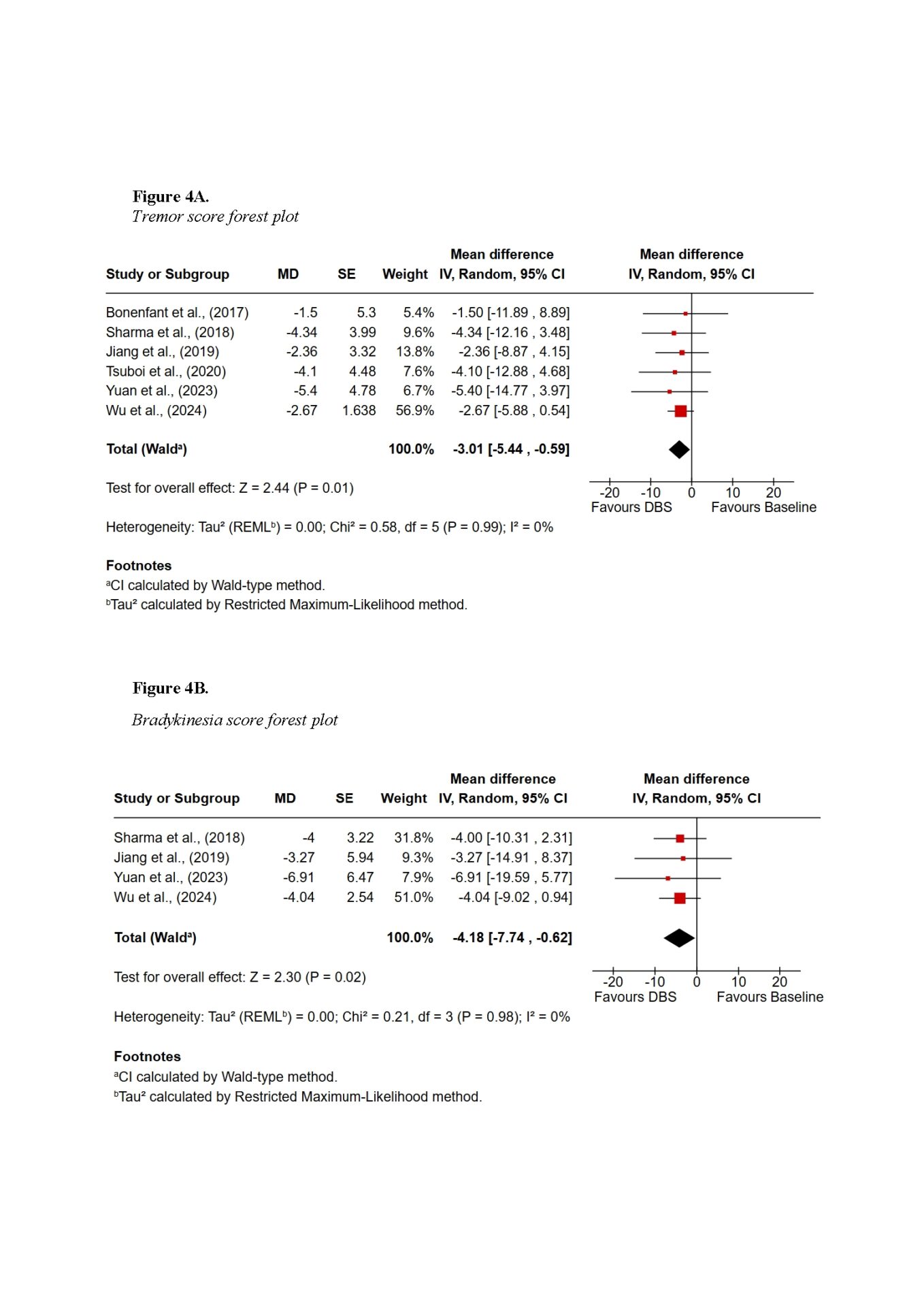
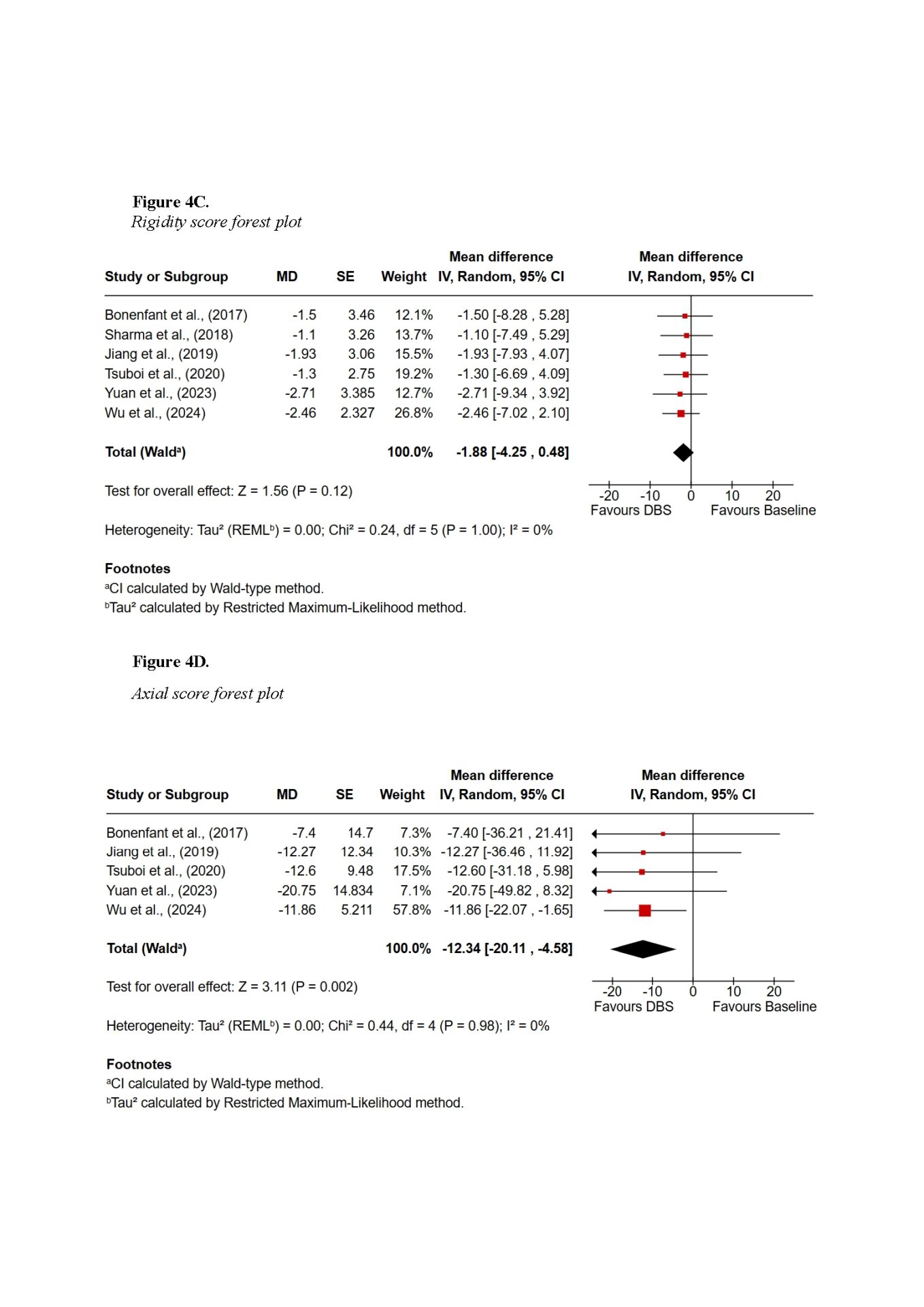
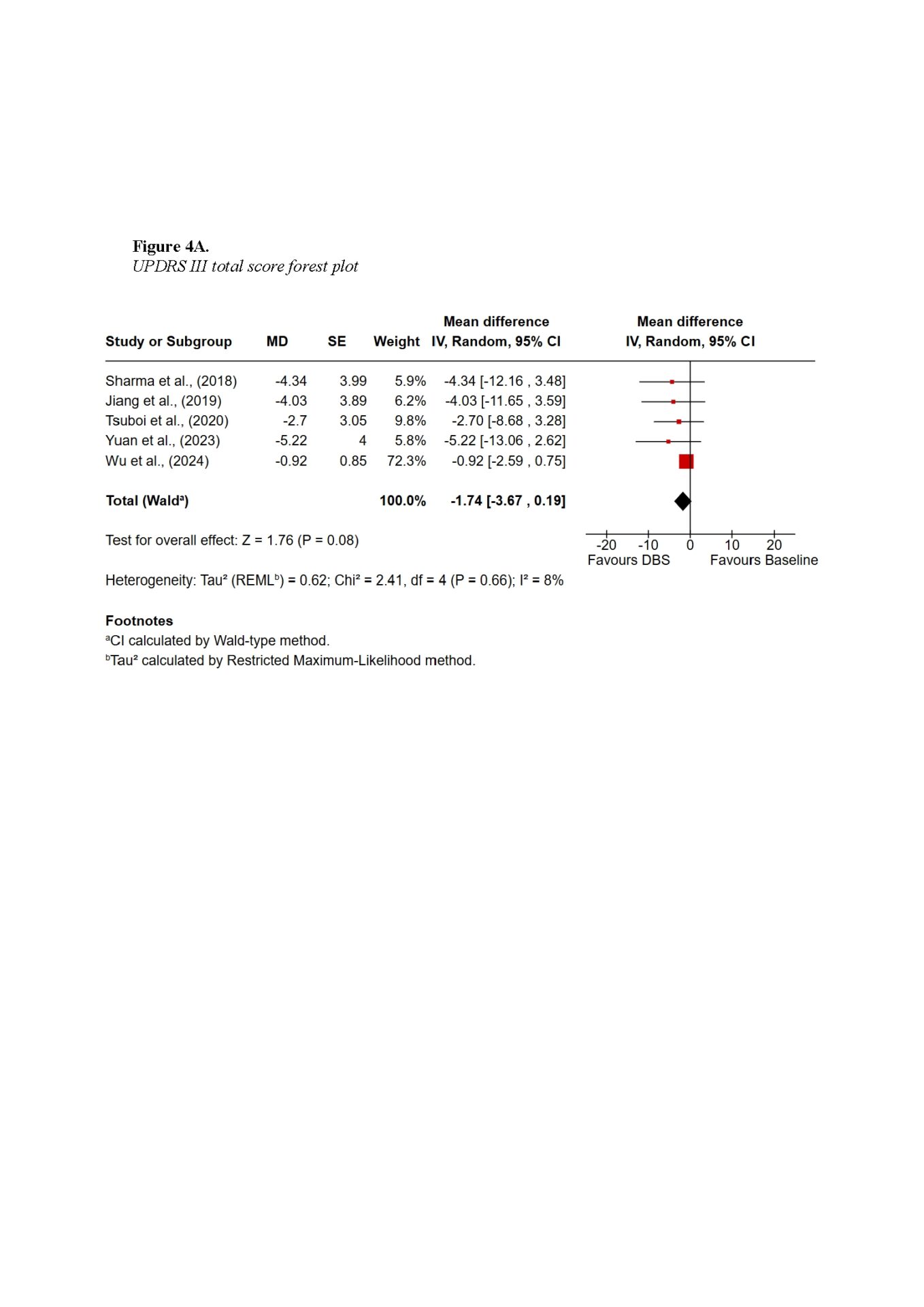
Findings demonstrated that DBS provides substantial global motor improvement, with tremor showing the most robust and consistent response across all subtypes. TD patients achieved the greatest overall benefit, with improvements exceeding 60% in some cohorts. AR and MX patients experienced intermediate benefits, while PIGD patients showed the least favourable outcomes, primarily due to the axial symptoms such as gait and postural instability. Subscore symptom domain meta-analysis also supported the highest improvements in tremor scores, with a pooled mean difference of −3.01 (95% CI −5.44 to −0.59; Z = 2.44, p = 0.01), whereas axial improvements did not reach statistical significance. Non-motor outcomes were more variable: TD patients tended to maintain cognitive stability, whereas PIGD patients were more vulnerable to postoperative cognitive decline. Quality-of-life improvements were evident across subtypes but differed in magnitude, reflecting baseline disparities.
In conclusion, DBS is an effective but selective intervention in PD, with outcomes strongly influenced by motor subtype and symptom domain. These findings highlight the importance of subtype-specific patient selection, careful preoperative counselling, and multidisciplinary management. Future research should prioritise standardised subtype definitions, systematic assessment of non-motor domains, and long-term follow-up to better inform personalised DBS strategies.