P-glycoprotein (PGP) and related efflux transporters reduce the intestinal absorption of therapeutic agents but may also have a key protective role in preventing the entry of potentially toxic xenobiotics from the gut lumen. To date, we have little direct information on the impact of PGP on the permeability of the epithelial barrier and, in particular, the functional distribution of PGP effects along the gut, which may give further insight into its physiological importance. To address this we have investigated the regional permeability of PGP substrates and permeability probes using intestinal tissues from mdr1a (-/-) mice, which do not express functional PGP in the intestine, and wild-type mdr1a (+/+) mice.
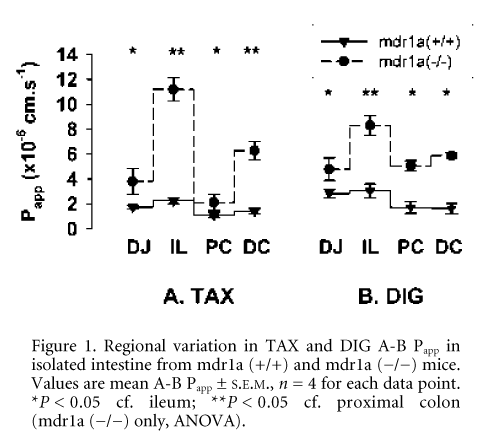
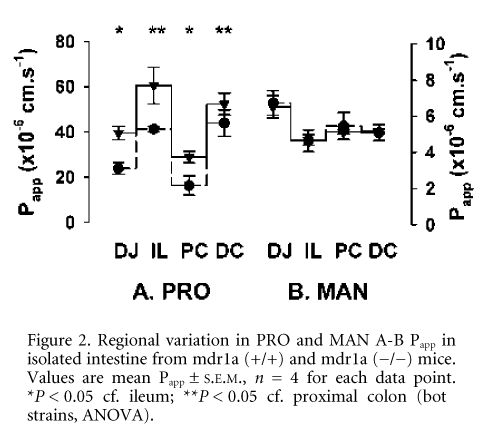
Unstripped distal jejunum (DJ), ileum (IL), proximal (PC) and distal colon (DC) was removed from humanely killed male FVB (mdr1a (+/+) or mdr1a (-/-)) mice, and mounted in modified Ussing chambers, using methods similar to those of Stephens et al. (2002). Apical to basolateral (A-B) apparent permeability (Papp) in each region was measured for the PGP substrates digoxin (DIG, 40 mM) and paclitaxel (TAX, 20 mM). Propranolol (PRO, 100 mM) and mannitol (MAN, 100 mM) were also used as markers of passive transcellular and paracellular permeability, respectively. All compounds were labelled with either [3H] or [14C] (0.2 mCi ml-1).
In mdr1a (+/+) intestine, the permeability of TAX (n = 4) and DIG (n = 4) was restricted, which was manifested as low absorption that varied little from jejunum to distal colon (Fig. 1). In contrast, active PGP-mediated efflux of TAX and DIG was abolished in mdr1a (-/-) tissues and this was accompanied by marked region-dependent increases in passive drug permeability (Fig. 1). Permeability of the passive transcellular marker PRO, whose transport is unaffected by PGP, showed similar regional variations in both mdr1a (+/+) and mdr1a (-/-) tissues (Fig. 2), indicative of inherent differences in transcellular permeability that are masked by differential expression of PGP. Permeability to the paracellular marker MAN was identical in both mouse strains and unlike PRO showed no regional variation. These data suggest that differential PGP expression along the gut maintains xenobiotic absorption at a low level and, in doing so, minimises regional variations in transcellular permeability.
This work was supported by Pfizer Global Research & Development.
All procedures accord with current UK legislation.