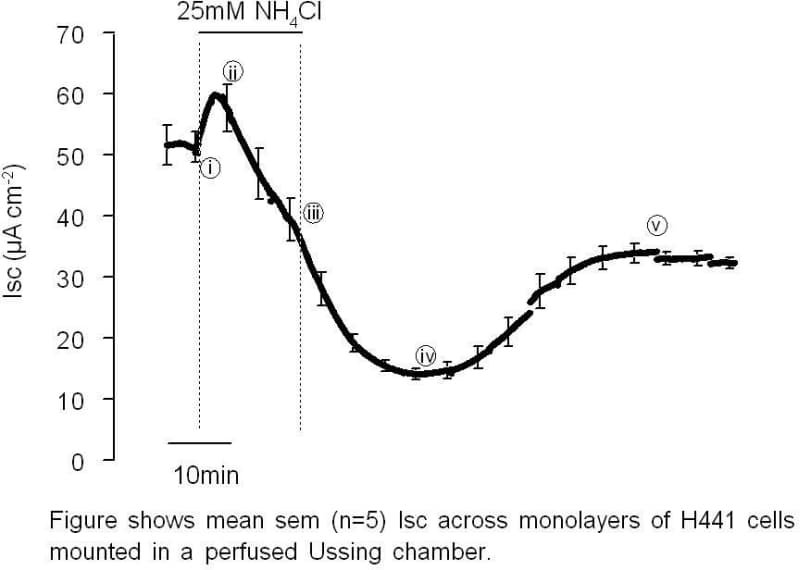
Changes in pHin have long been known to alter the rate of Na+ transport across Na+-transporting tight epithelia (1, 3). The aim of this current study was to investigate the effect of changes in pHin on Na+ absorption across H441 cells, a Na+ absorbing human airway epithelial cell line. Cells were grown to confluence on Snapwell filters (Costar) and mounted in Ussing chambers (1cm2 exposed surface area, chamber volume 1-1.5ml). The apical and basolateral chambers were then continuously perfused with HCO3– -buffered Krebs solution (37°C, ~1ml min-1). Cells were maintained under open circuit conditions until transepithelial potential difference (PD) had stabilised (15-30min). Mean PD, short circuit current (Isc) and transepithelial resistance (Rt) under those conditions were 16.6±2.8mV, 54.5±6.0μAcm-2 and 292±22Ωcm2 respectively (n=6). Transepithelial PD was then clamped at 0mV using a voltage clamp and the current required to maintain this PD (Isc) was continually monitored and recorded to computer disk. Ammonium chloride (25mM) was added to both sides of the epithelium to alkalinise pHin and this induced an initial rise in Isc (i-ii in Figure). Isc then began to fall, presumably as pHin began to acidify (ii-iii), before subsequent removal of NH4Cl, which acidifies the cells, induced a large inhibition of Isc (iii-iv). Isc then slowly recovered (iv-v), presumably as the cellular pHin regulatory mechanisms returned pHin to normal levels. Addition of apical amiloride (10μM) inhibited this effect of changing pHin on Isc, suggesting that the effects are mediated by a change in Na+ transport. Addition of 5-(N-Ethyl-N-isopropyl) amiloride (EIPA; 100μM), a Na+/H+ exchange inhibitor, had no effect on either the initial rise in Isc or the subsequent fall but did block the recovery of Isc towards the basal level (iv-v). Presumably it inhibited the recovery of pHin from acidosis and thus the Isc remained depressed. In summary, alkalinisation stimulates Na+ absorption across H441 airway epithelial cells, whilst acidification inhibits Na+ transport. These results show that in common with Na+ transport across tight epithelia (1-3) Na+ absorption in airway epithelia is acutely dependent on pHin.
University of Bristol (2005) J Physiol 567P, PC170
Poster Communications: Regulation of Na+ transport in airway epithelia by intracellular pH (pHin)
Constable, Maree; Inglis, Sarah;
1. Maternal and Child Health Sciences, University of Dundee, Dundee, United Kingdom.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.