Introduction: Recent investigations suggest that acute exercise decreases Ca2+, subsequently stimulating parathyroid hormone (PTH) and bone breakdown (Townsend et al., 2016; Kohrt et al., 2018). It has been suggested the exercise-induced decrease of Ca2+ may contribute to the low bone mineral density phenomenon observed in many endurance athletes (Duckham et al., 2012; Scofield and Hecht, 2012). The aim of this study was to investigate the effect of exercise intensity, and the associated acid-base changes, on Ca2+ and PTH.
Methods: Twelve healthy males (n = 12) completed a three-arm, randomised-counterbalanced design experiment. Physiological thresholds and associated workloads were identified from a maximal cardiopulmonary exercise test. Participants completed 30-minutes (or until volitional fatigue) of cycle exercise below gas exchange threshold (GET), above GET, or 10W above the estimated critical power (Above RCP). Blood samples were taken every 5 minutes for 35 minutes, or until volitional fatigue. Ca2+ and pH were analysed using an i-STAT point of care device and EG7+ cartridge system. PTH was analysed using ELICA. Data were analysed using linear mixed effects models and omnibus ANOVAs of fixed terms (R Core Team, 2020).
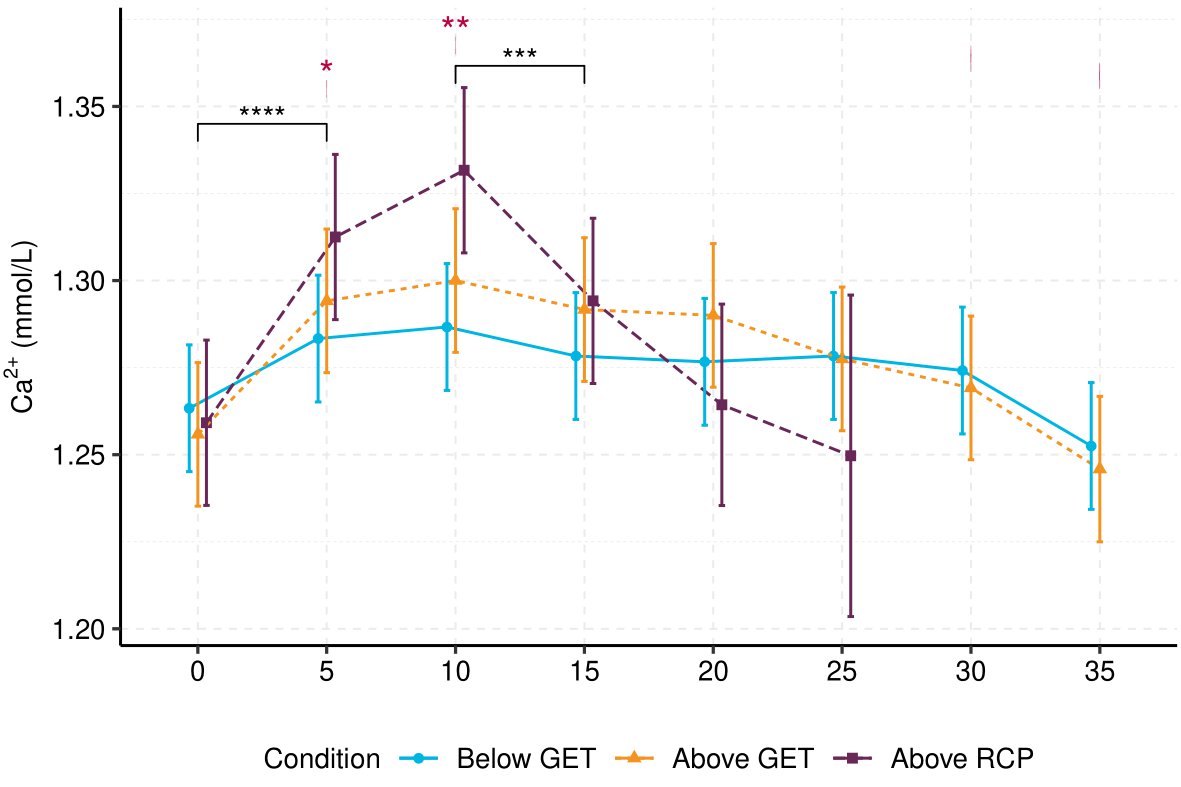
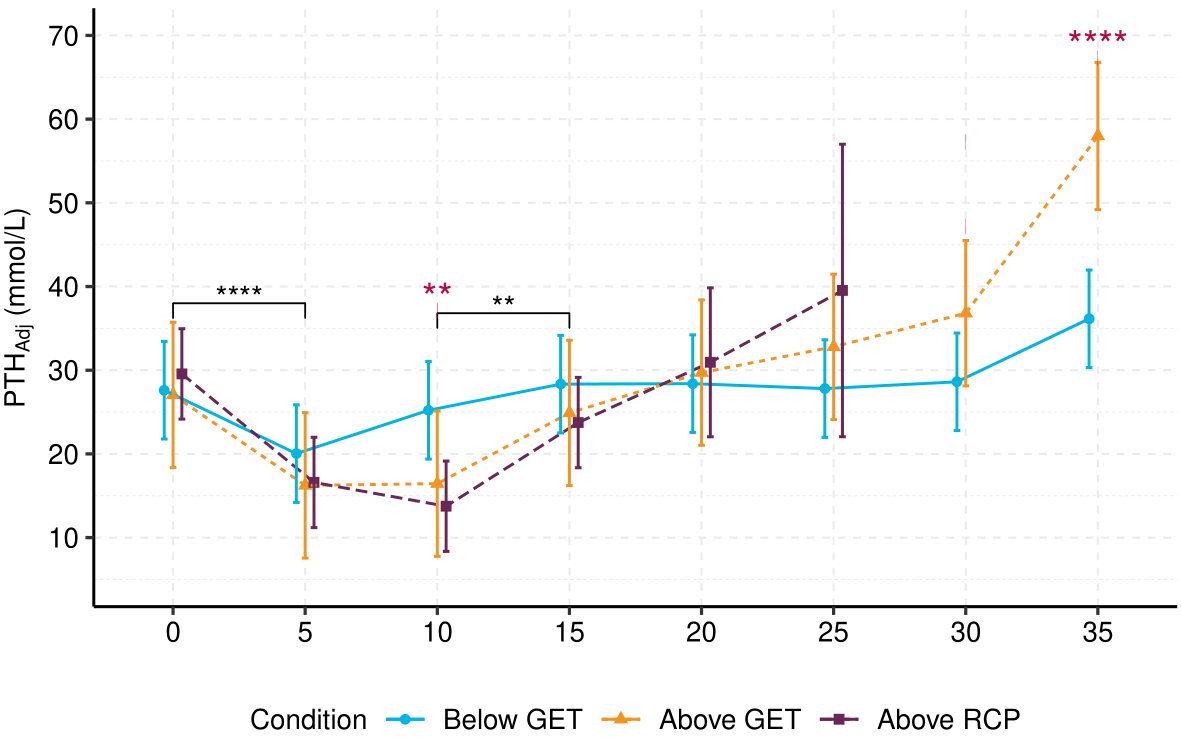
Results: Exercise produced a biphasic intensity-dependent Ca2+ response to exercise (F (12, 189.83) = 3.45, p <.001). Exercise below GET did not alter Ca2+ when referenced to baseline (Mdiff = -0.01–0.02 mmol⋅L-1, SE = 0.01, p > 0.05), but exercise above GET (including above RCP) significantly increased Ca2+, peaking at 10-minutes (Mdiff = 0.04–0.07, SE = 0.01, p < 0.001). Plasma-volume adjusted PTH (PTHAdj) was significantly decreased in the initial 10-minutes of exercise above GET/RCP (Mdiff = -15.81 – -10.61 , SE = 3.30, p < 0.001). Ca2+ decreased throughout the remainder of exercise above GET and RCP, returning to baseline concentrations. PTHAdj mirrored Ca2+’s response: PTHAdj increased from 10 minutes above GET and above RCP, but was only significantly greater than baseline following above GET exercise (Mdiff = 30.93, SE= 3.38, t(189.33) = 9.14, p < 0.001). Introducing pH as a covariate in the Ca2+ model (b = -0.27, SE = 0.06, t(189.30) = -4.11, p < 0.001) removed significant interactions at 5 and 10-minutes between exercise below GET and above RCP. However, pH could not account for all Ca2+ variation, as the main effect of time (F (7, 191.77) = 13.85, p < 0.001), and its interaction with condition (F (12, 190.17) = 4.37, p < 0.001), remained significant. Pooled concentrations of PTHAdj were negatively associated with Ca2+ (R = -0.6, p < 0.001).
Conclusion: These findings suggest GET may act as an intensity threshold for eliciting significant biphasic response in Ca2+ and PTHAdj. pH may explain the intensity-dependency of Ca2+ during exercise, likely due to the physiochemical competitive binding model (Pedersen, 1972; Fogh-Andersen et al., 1993). However, pH could not account for the entirety of Ca2+’s temporal response, suggesting other exercise-mediated effects may play a role in calcium regulation. It appears Ca2+ is important for mediating PTH response to exercise, but that other exercise-induced responses may also influence PTH and subsequent bone metabolism.