Background: Missions into Deep Space (DS) are planned for the next two decades; a permanent orbital lunar station by end of the 2020s and first manned trip to Mars by late 2030s. The biology of kidney function in spaceflight has been largely overlooked. We suspect that kidney physiology may be disturbed in spaceflight, either by microgravity (MG) and/or Galactic Cosmic Radiation (GCR) potentially leading to kidney injury and stone formation.
MG exposed astronauts have unusually high rates of kidney stones. Indeed, MG associated changes in urinary biochemistry favour stone formation, and current thinking suggests this is secondary to bone demineralisation due to unloading. However, the compartmental fluid shifts associated with MG cause changes in differential perfusion pressures in tissue beds and changes in baroreceptor activation. We predicted this would cause physiological changes in renal sodium and calcium transporter expression (which tend to be physiologically linked), resulting in elevated urinary calcium loss, kidney stone formation and decreased bone mineral density as kidney-driven primary events.
The kidney is an exquisitely radiation sensitive organ; it is the dose limiting organ in abdominal radiotherapy. Chronic kidney dysfunction can occur with acute low linear energy transfer (LET) radiation doses as low as <0.5 Gy; the LET dose expected on a Mars Mission. However, GCR is comprised of low-LET x- and γ-rays, protons and high energy ions of heavier elements 'HZE' (e.g. iron). We hypothesise that GCR may cause renal damage within the timeframe and dose expected for a mission to Mars.
Methods: We performed biomolecular (Epigenomic, bulk and spatial mRNA and miRNA transcriptomic, proteomic, phosphoproteomic, metabolomic, metagenomic), clinical chemistry (electrolytes, endocrine and biochemistry biomarkers) and imaging (histology, immunofluorescence, miRNA FISH, 3D morphometry) analyses using samples and datasets available from 11 spaceflight-exposed mouse (13-75 days on ISS/shuttle) and 5 human (3-180 days on ISS/Shuttle) collections of missions, and 4 simulated GCR-exposed mouse experiments (0.5-0.75Gy ≈ 1.5-2.5-year dose / necropsy 1-180 days post-exposure). In total the study encompassed approximately ~100 humans / ~155 mice (n per group). All animal and human studies had local ethical committee approval and licenses where appropriate.
Results: Historical human data showed hypercalciuria, hyperphosphaturia and reduced urine volume during spaceflight. Multiomic pathway enrichment analysis indicated changes in kidney gene products associated with nephrolithiasis. MG-exposed animals had significant dephosphorylation of thiazide-sensitive SLC12A3 and furosemide-sensitive SLC12A1 canonical transporters known to impact nephrolithiasis and bone mineral density, as well as dysbiosis of faecal microbial genera known to influence nephrolithiasis.
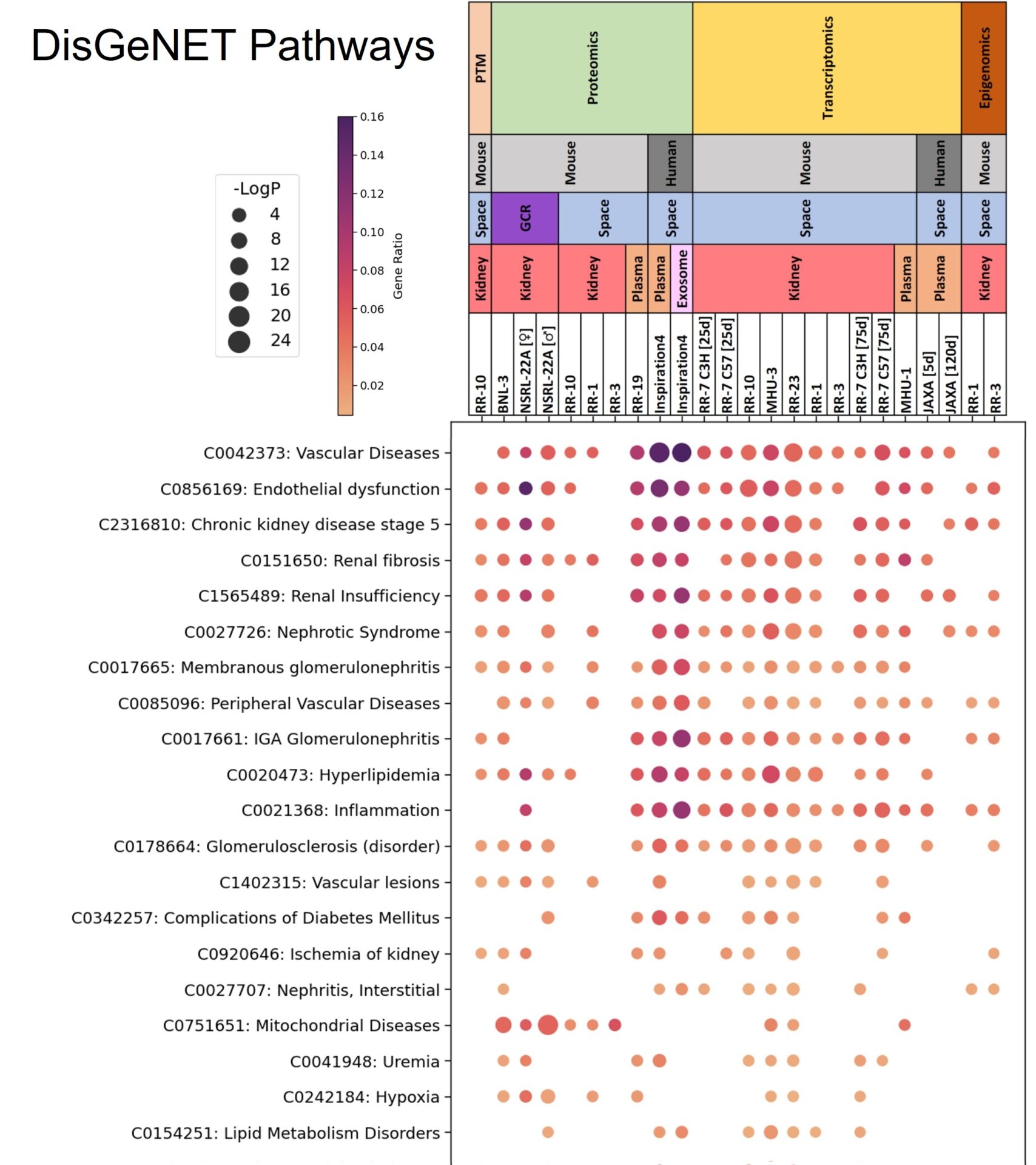
Pathway analysis of human and mouse plasma and kidney samples showed enrichment of gene products associated with renal fibrosis, inflammation, glomerular and tubular injury (Fig.1). GCR-exposed animals had decreased mitochondrial protein expression, and elevation in pathogenic miRNA species. Spatial transcriptomics revealed immune cell infiltration of the cortex, biochemistry revealed elevated urinary protein loss and histopathological investigation revealed incidences of thrombotic microangiopathy.
Conclusion: Our data provide strong evidence that prolonged exposure to DS will induce significant harm to renal health and function that may prove mission critical. Development of mitigation strategies will be essential for long-term missions to the moon, mars and beyond.