Sepsis is a life-threatening host reaction to circulating pathogens associated with reduced microperfusion and tissue hypoxaemia. Thrombocytopenia, one of the central diagnostic criteria for sepsis, is a distinct negative prognostic marker for survival (1, 2). Thrombocytes capability has transcended, solely within the coagulation system, to encompass a modulatory role in the immune response and a direct mediator of pathogen killing. Studies has shown a binding capability among others to monocytes, neutrophils and pathogens (3-5). Interestingly, our preliminary data in a murine model of sepsis reveal that the thrombocyte number falls prior to intravascular coagulation. Here we investigate the fate of circulating thrombocytes during sepsis.
All experiments were carried out in male Balb/cJRj mice (8-10 weeks, 24.8 ± 0.2 g). Mice were anaesthetised for the entire experiment with ketamine/xylazine (100/10 mg kg-1) sc. A bolus of 330∙106 E. coli (O6:K13:H1) with or without eGFP-pBAD plasmid was administered through the tail vein. The number of circulating thrombocytes (CD42+), neutrophils (Ly6G+) and monocytes (Ly6C+/CD11b+) were determined by flow cytometry. Bacterial load was quantified as CFU on LB-agar plates or by flow cytometry (EGFP). The results are given as mean±S.E.M., and statistical significance was tested by two-way ANOVA in GraphPad Prism v10.2.1 Experiments were approved by the National Animal Experiment Expectorate, Denmark (2020-15-0201-00422).
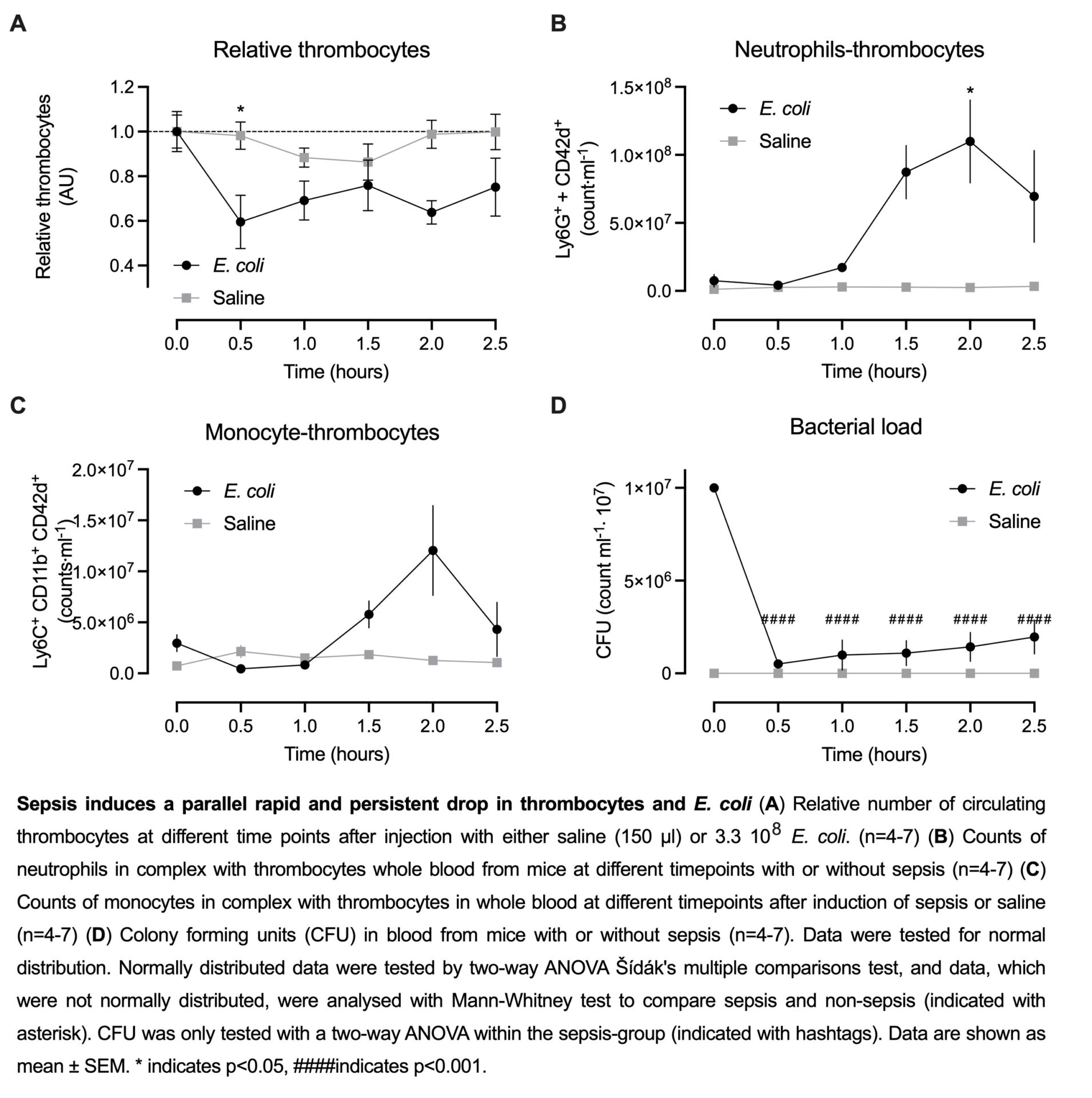
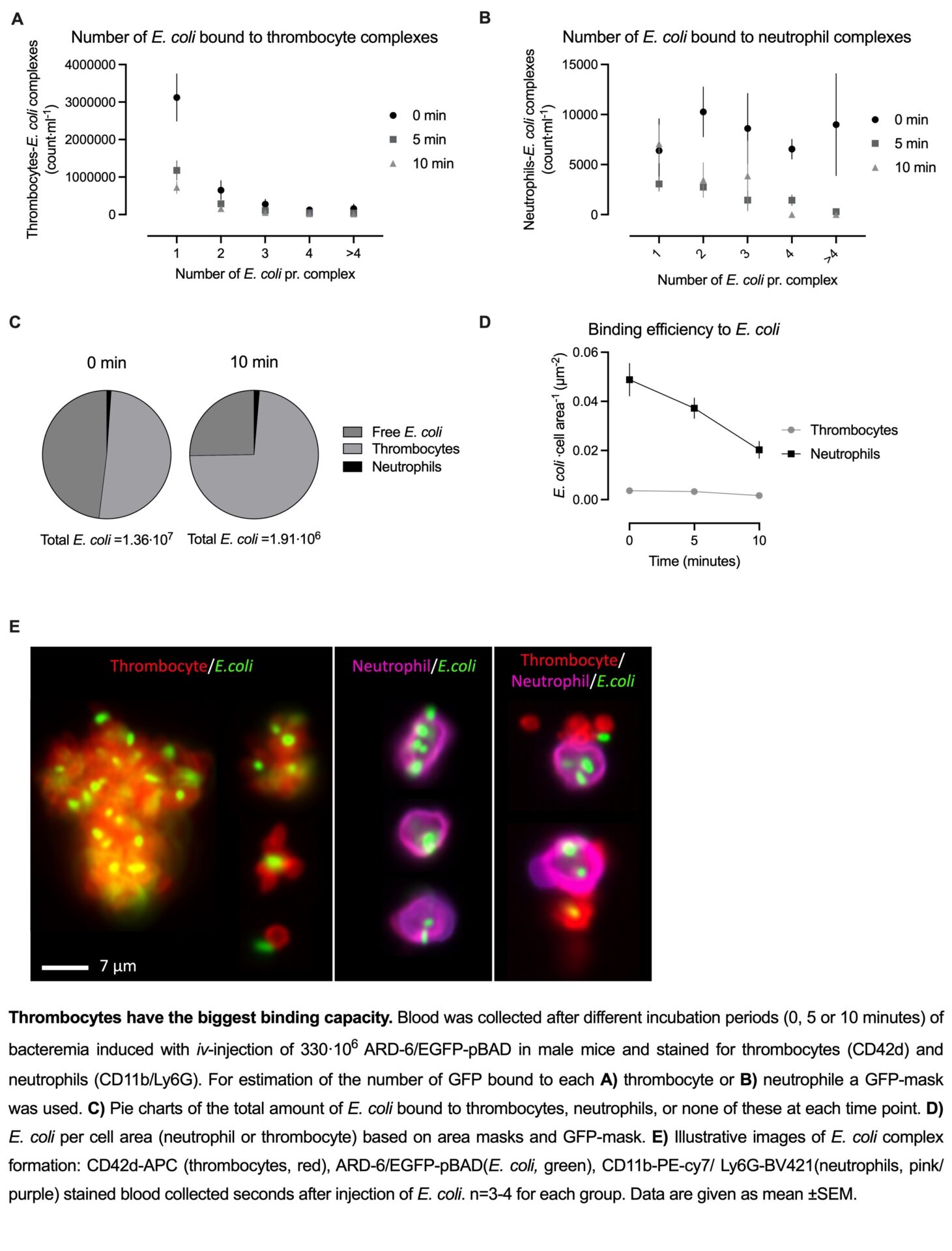
In this model, sepsis-induced thrombocytopenia develops in a biphasic manner. We detected an early reduction of about 38.6%, already 30 min after E. coli injection (48.9⋅107±97.8⋅106 thrombocytes/ml, n=6) compared to mice receiving vehicle (77.4⋅107±48.2⋅106 thrombocytes/ml, n=6 p<0.06). Thrombocytes are known to form complexes with neutrophils or monocytes. However, the number of thrombocytes in complex with neutrophils or monocytes remained constant during the early fall in thrombocyte number and, thus, cannot explain the early drop in circulating thrombocytes. Interestingly, we found that the number of bacteria in the blood fell in parallel with the thrombocytes from 1.0⋅107±0⋅107CFU (n=4) immediately after injection to 5.1⋅105±0.9⋅105 CFU (n=7) 30 minutes after injection. By image-enhanced flow cytometry, we were able to show that the EGFP-expressing uropathogenic E. coli instantly and primarily is scavenged by circulating thrombocytes and that these complexes are acutely removed from the circulation. Preliminary in vitro data support the theory of thrombocytes acting primary as a scavenger system during sepsis. Data indicates that thrombocytes possess the ability to induce bacterial death, albeit gradually, but not sufficiently pronounced to achieve a 94.9% reduction in bacterial load within 30 min.
The data strongly suggest that circulating thrombocytes constitute the most important cell type for fast scavenging and clearance of invading bacteria during urosepsis.