Physiology News Magazine
A clinical perfusion scientist:
The job and the role in ECMO during the COVID-19 pandemic
Membership
A clinical perfusion scientist:
The job and the role in ECMO during the COVID-19 pandemic
Membership
Naoise Ó Ciardha, Lead Clinical Perfusionist, Royal Infirmary of Edinburgh, UK
Kalynne Royds, Senior Clinical Perfusionist, Royal Brompton Hospital, London, UK
https://doi.org/10.36866/pn.121.38
“Percussionist? Perfumist?”
Admittedly, the rhythmic pulsing of an ECG trace could sit comfortably alongside the timpani or xylophone but the scent of freshly cauterised fat against a backdrop of iodine and alcohol hand-gel is not something I would bottle. Once you get past the pronunciation, explaining the job of a perfusionist is slightly easier.
A perfusionist operates the heart and lung machine for patients undergoing cardiac surgery. Venous blood is drained from the right side of the heart via a large cannula into the cardiopulmonary bypass circuit of the heart and lung machine. Blood is pumped through an artificial lung called an oxygenator where gas exchange occurs. Oxygenated blood is then pumped into the ascending aorta to follow its normal course. The heart and the lungs are now isolated from circulation (i.e., cardiopulmonary bypass), providing a controlled environment for surgical intervention.
Indeed, opening the heart for valve repair or replacement requires it to be still. The perfusionist delivers a high potassium solution called cardioplegia to the coronary circulation, which causes an arrest – paradoxically, this same solution used for lethal injection protects the heart in this setting. Eliminating the heart’s electrical activity and keeping it cool substantially reduces the metabolic rate of the myocardium, effectively putting it into a state of hibernation whilst ischaemic (Gay & Ebert, 1973). Once re-perfused with blood, resting membrane potential of the myocytes is restored and contraction can occur. This Lazarus moment of transition from a motionless mass to a dynamic organ is testament to the remarkable resilience of the heart.
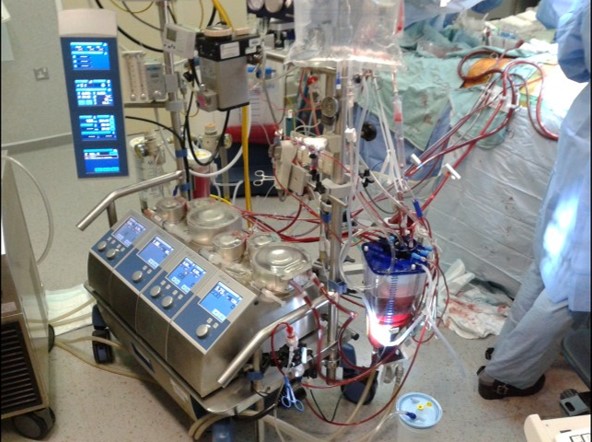
During bypass, perfusionists must maintain haemodynamic stability, balance electrolytes through blood gas management, and monitor the coagulation status, all whilst ensuring the cardiopulmonary circuit is functioning correctly. An intricate knowledge of physiological processes is essential to manipulate the parameters of bypass to maximise not just O2 delivery but whole-body homeostasis. For example, mimicking hypoxia by increasing the partial pressure of CO2 can mitigate shunting of blood through aortopulmonary collateral vessels by causing them to constrict (Sakamoto et al., 2004). Also, transient reductions in pump flow which impacts O2 delivery can be tolerated if core body temperature is reduced (Cook, 1999).
Since the first successful use of cardiopulmonary bypass by Dr John Gibbon in 1953, extra-corporeal technology has evolved and become more refined, as has the profile of the person behind the pump. The perfusionist has shifted from an auxiliary to a highly specialised role with an MSc in Perfusion now required to practise in the UK and Ireland. Perfusionist roles also include coagulation management and blood conservation through the operation of cell salvage machines and point-of-care testing. With expertise in fluid dynamics, responsibilities also involve other forms of extracorporeal life support (ECLS) like ventricular assist devices and extracorporeal membrane oxygenation (ECMO).

ECMO is an advanced life-support that can be used in patients with cardiac and/or respiratory failure. The device is a miniaturised circuit capable of blood oxygenation, CO2 removal, and haemodynamic support (Ali and Vutlsteke, 2019). Perfusionists are responsible for safe setup and deployment of ECMO, and maintenance and troubleshooting during use. Throughout the COVID-19 pandemic, ECMO has proven to be life-saving for many when conventional ventilatory intervention is not enough. SARS-CoV-2 can induce severe respiratory distress, subsequent failure of adequate blood oxygenation, and lead to global systemic hypoxia, hypercapnia, and organ failure. This can even occur despite invasive mechanical ventilatory support. ECLS has been essential in providing oxygenated blood to those with failing lungs. With ECMO, struggling lungs can recover without the risk of pressure-induced lung trauma from the ventilator.
The demand for ECLS has grown with the course of the pandemic. Specialised retrieval teams – comprising a critical care intensivist, a specialised nurse, and a perfusionist – have been mobilised to implement ECLS in severely affected patients and aid their transport to specialist hospitals with ECLS units, including the Royal Brompton Hospital in London. Currently, the ECLS unit at the Royal Brompton can accommodate 25 ECMO-equipped beds. This is one of the largest units in England. While 25 may seem a small number, the number of staff and staffing hours required to provide round-the-clock care is high. Add in a year-long battle and a near permanent daily requirement of stifling personal protective equipment and it becomes a challenge of even greater proportion.

Unfortunately, the highly specialised and invasive nature of ECLS means supply does not meet current national or global demand and candidates must be screened for suitability. Furthermore, its implementation is not without significant risk to patients. Being artificial, anticoagulation of the patient’s blood is necessary to prevent obstruction of the ECMO circuit; thus, imposing significant risk of bleeding. A delicate balancing act of preventing thrombosis and ensuring adequate haemostasis is required throughout ECLS intervention (Zhang and Zhou, 2021). Current data indicate mortality rates of up to 38% for COVID-19 patients in the 90 days post-ECLS intervention (Barbaro et al., 2020). One of the primary concerns when considering a suitable candidate is the reversibility of their pathological state and their ability to regain physiological independence when removed from the ECMO circuit.
The greatest burden to the ECLS team during the pandemic is deciding who is and who isn’t a candidate for ECMO. However, the decisions surrounding implementation of ECLS and best patient care has encouraged discussion amongst multidisciplinary teams and boosted medical ingenuity and quick progress in COVID-19 treatment modalities.
References
Ali J and Vuylsteke A (2019). Extracorporeal membrane oxygenation: indications, technique and contemporary outcomes. Heart 105(18), 1437–43.
Barbaro P et al. (2020). Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. The Lancet 396(10257), 1071–78.
Cook DJ (1999). Changing temperature management for cardiopulmonary bypass. Anesthesia & Analgesia 88(6), 1254–71.
Gay WA and Ebert PA (1973). Functional, metabolic, and morphologic effects of potassium-induced cardioplegia. Surgery 74(2), 284–90.
Sakamoto T et al. (2004). The influence of pH strategy on cerebral and collateral circulation during hypothermic cardiopulmonary bypass in cyanotic patients with heart disease: results of a randomized trial and real-time monitoring. The Journal of Thoracic and Cardiovascular Surgery 127(1), 12–19.
Zhang Y et al. (2021). ECMO support for COVID-19: a balancing act. The Lancet 397(10269), 94–95.