
Physiology News Magazine
Astrocytes and hepatic stellate cells (HSCs): Stars of tissue regeneration
Astrocytes and HSCs in tissue regeneration
Features
Astrocytes and hepatic stellate cells (HSCs): Stars of tissue regeneration
Astrocytes and HSCs in tissue regeneration
Features
https://doi.org/10.36866/pn.128.24

Krystal English
Medical Student Researcher, Department of Cancer Biology, University of Texas MD Anderson Cancer Center, US

Caleb Johnson
Postbaccalaureate Student, Department of Cancer Biology, University of Texas MD Anderson Cancer Center, US

Professor Raghu Kalluri
Department of Cancer Biology, University of Texas MD Anderson Cancer Center, US
Hepatic stellate cells (HSCs) in the liver and astrocytes in the brain have much more in common than just their beautiful star-shaped appearance. In this feature, we highlight the intriguing parallels between these cells that populate two very different organs: the liver and the brain. Both cell types contribute to maintaining a blood–tissue barrier and transdifferentiate to a myofibroblast phenotype in response to injury to regenerate damaged tissue. This adaptive response can become maladaptive with continued or chronic injury, leading to fibrosis that eventually disrupts the ability of the organ to function normally. Glial fibrillary acidic protein (GFAP) is one known marker for the activation of both HSCs and astrocytes, further highlighting functional similarities in their response to cellular damage between the two cells. Liver health and brain health are functionally connected in ways that go beyond the analogies between these two cell types.
HSCs are the guardians of liver health
The liver is a key organ that controls digestion, toxin removal, retinoid (vitamin A) storage and metabolism, and the synthesis of the mediators of the coagulation cascade. It is well established that the liver can regenerate, and hepatic stellate cells (HSCs) drive the process of liver regeneration. HSCs are a representation of mesenchymal stem cells that make up 5%–8% of the cells in the liver (Higashi et al., 2017). They are located in the space of Disse (perisinusoidal space), located between the hepatocytes and the tubular sinusoids that allow blood flow. HSCs possess extensions that wrap around the sinusoids and facilitate communication with sinusoidal endothelial cells, hepatic epithelial cells, hepatocytes, and Kupffer cells (resident liver macrophages) (Wake, 2006).
HSCs are normally quiescent, and control sinusoidal tone and blood flow, hepatocyte proliferation, angiogenesis and maintenance of the extracellular matrix (ECM). They undergo activation in response to acute injury, upregulating smooth muscle α-actin and transdifferentiating into myofibroblasts (Kitto and Henderson, 2021). Chronic damage such as that caused by alcohol impacts hepatocyte mitochondria, causing cell death and also activating the conversion of HSCs to myofibroblasts.

Like astrocytes, HSCs show increased expression of glial fibrillary acidic protein (GFAP) during the process of transdifferentiation. GFAP is part of both HSC and astrocyte intermediary fibre systems that acts as a “command centre” for responding to cellular stress (Hol and Pekny, 2015). During chronic injury, various factors are released by HSCs including transforming growth factor beta (TGF-β), collagen (type I, III, and IV) and other ECM proteins (Kitto and Henderson, 2021). Repeated or chronic injury leads to sustained increase in expression of ECM components, resulting in scarring (Higashi et al., 2017). In the case of acute injury, the ECM “scar” has the opportunity to resolve and regenerate, but in chronic injury (e.g. alcoholic liver disease, non-alcoholic fatty liver disease, untreated hepatitis) the scar becomes dense and fibrotic (Fig.1). The pathologic scar creates a barrier against the circulation of growth factors, creating a poor environment for hepatocyte proliferation. The vascular network also undergoes scarring leading to irregular blood flow and prevention of necessary nourishment (i.e. Hepatocyte Growth Factor [HGF]). Eventually, the fibrotic scarring forms fibrotic nodules, where extensive fibrotic tissue creates visible raised tissue, resulting in cirrhosis that can be detected on a CT scan.
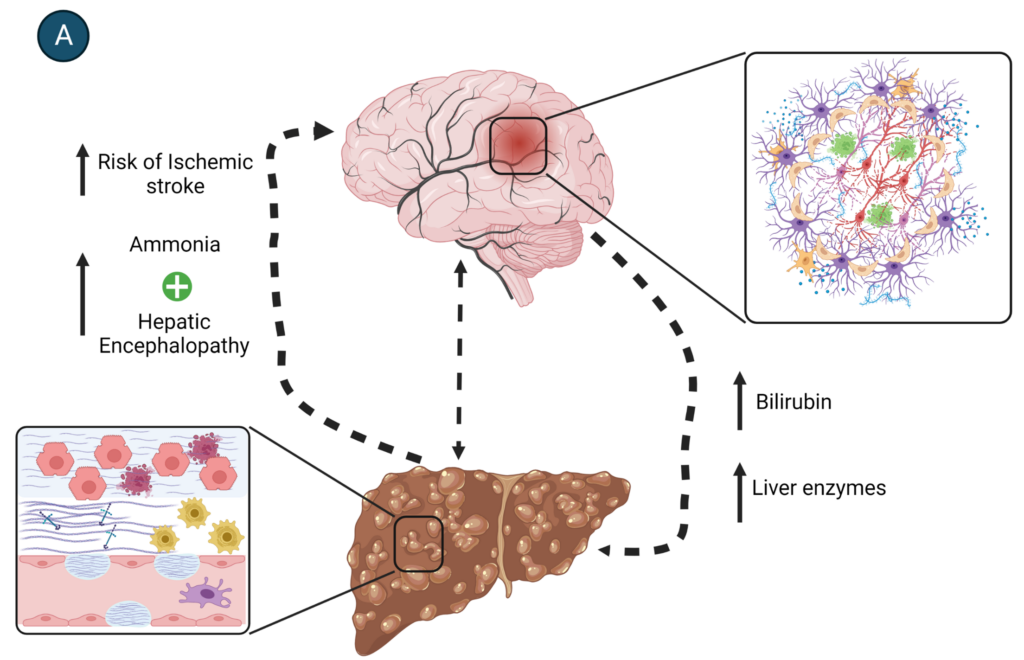
With cirrhosis, liver function is decreased and the patient experiences symptoms that include coagulopathy with an associated risk of ischaemic infarction, malnutrition, and increase in blood toxin (e.g. ammonia) concentration. Excess ammonia can lead to systemic effects, with the brain being one of the most sensitive to this toxin. Liver dysfunction is one of the major causes of toxic encephalopathy.
Astrocytes are the guardians of brain health
Astrocytes are glial cells that originate from neural stem cells (NSCs) in the central nervous system (CNS). They are the most abundant cell type in the CNS, making up 20%–40% of glial cells, which constitute close to 90% of the cells in the CNS. Astrocytes are found throughout the brain, especially around blood vessels of the brain and the spinal cord (English et al., 2020). Like HSCs, astrocytes use long cell processes for communication and nutrient transport between cells, including neurons, other glial cells, and vascular epithelial cells. Astrocytes provide physical and chemical support for neuronal growth from stem cell to maturity, synaptic maturation, and neurotransmitter regulation including protection against neurotransmitter toxicity, vascular tone regulation, metabolic regulation via ammonia metabolism, and protection from foreign pathogens and drugs as part of the blood brain barrier (English et al., 2020).
Like the liver, the brain has the ability for cellular repair and regeneration, mediated by the astrocytes in the CNS. In response to damage, such as that caused by stroke and toxins, astrocytes donate their mitochondria to damaged neurons for cellular repair (English et al., 2020) and release factors like Olig2 to promote oligodendrocyte precursor cell (OPC) development into mature oligodendrocytes (Weber et al., 2021). Astrocytes can act as NSCs and mature cortical astrocytes can undergo “reprogramming” to NSCs in the presence of injury (e.g. experimental ablation) (Gonzalez-Perez and Quiñones-Hinojosa, 2012). Finally, similar to HSCs, astrocytes create a scar called “glial scar” in response to injury.

On sensing injury, astrocytes become reactive and begin to express GFAP, a cytoskeletal intermediate filament (IF) (English et al., 2020). This is accompanied by cytokine (i.e. TGF-α, Il-6) release from astrocytes, and also from other glial cells and neurons. Reactive astrocytes and perivascular fibroblasts proliferate and surround the cellular lesion using elongated processes to form a glial scar (Fig.2). The glial scar is a physical and functional wall around the necrotic brain tissue, serving to wall off toxic necrotic induced pro-inflammatory cytokines (English et al., 2020). Generally, this impacts function that the specific area of the brain controls (e.g. a cerebellum infarct would impact motor control). As the glial scar persists, it becomes a double-edged sword, not only preventing the spread of necrosis-induced mediators from dying cells, but also inhibiting regeneration and recovery of neuronal synapses and function (Aktas et al., 2007; English, 2020; Yang et al., 2020). The inhibition is driven by the production of ECM components such as chondroitin sulfate proteoglycans (CSPGs), which actively repel neuronal axon growth as well as OPC maturation (Yang et al., 2020). Even if the glial scar did not impede neural stem cell migration and differentiation, this does not necessarily mean that the new neuron would differentiate into the correct neuron population or have appropriate synaptic strength necessary for survival as the environmental cues for this process are missing. Using NSC transplantation as therapy for acute (<7 days) ischaemic infarcts has had mixed success. Though NSCs travel to neuronal circuits and can integrate into the circuitry they do not survive for long, and it is unknown if they possess the appropriate cellular phenotype that existing neurons do (Baker et al., 2019).
The importance of liver function in brain health
HSCs and astrocytes are the stars of liver and brain health, each playing a key role in cellular/tissue repair. They are, however, also functionally connected in less obvious ways. Specifically, the systemic consequences of liver disease have a devastating impact on brain health. This connection is exemplified by the higher incidence of hepatic encephalopathy (HE) and stroke in patients with liver cirrhosis.
HE is a neuropsychiatric syndrome that is the result of brain dysfunction and is caused by alcoholic liver disease (Fig.3). The incidence of HE in alcohol-induced cirrhosis is roughly 45%–50%. The processing of alcohol in the liver produces highly toxic chemicals like ammonia, triggering inflammation that leads to liver cell death. Excessive amounts of ammonia overwhelm astrocytic metabolism leading to dysfunction and glutamate toxicity, causing neuronal damage and/or death.
Cirrhosis can also cause coagulopathy, hypoperfusion, impaired glucose metabolism, and dyslipidaemia, all shown to be risk factors for stroke. The incidence of stroke, both haemorrhagic and ischaemic, is increased in patients with liver disease.
Conclusions
HSCs and astrocytes resemble each other in both structure and function. Their cellular features reflect the analogous functional roles they play in the liver and brain. Importantly, liver disease takes a heavy toll on organs other than the liver. The brain is particularly sensitive to the systemic effects of liver disease, and liver disease should be included as a differential diagnosis for patients with mental confusion and behavioural changes.
References
Aktas et al. (2007). Neuronal damage in brain inflammation. Archives of Neurology 64 (2), 185–189. https://doi.org/10.1001/ARCHNEUR.64.2.185
Baker EW, Kinder HA, West FD (2019). Neural stem cell therapy for stroke: A multimechanistic approach to restoring neurological function. Brain and Behavior, 9 (3). https://doi.org/10.1002/BRB3.1214
English et al. (2020). Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathologica Communications 8 (1):36. https://doi.org/10.1186/s40478-020-00897-7
Gonzalez-Perez O, Quiñones-Hinojosa A. (2012). Role of astrocytes as neural stem cells in the adult brain. Journal of Stem Cells 7 (3), 181. https://doi.org/jsc.2012.7.3.181
Higashi T, Friedman SL, Hoshida Y (2017). Hepatic stellate cells as key target in liver fibrosis. Advanced Drug Delivery Reviews 121, 27–42. https://doi.org/10.1016/J.ADDR.2017.05.007
Hol EM and Pekny M (2015). Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Current Opinion in Cell Biology 32, 121–130. https://doi.org/10.1016/J.CEB.2015.02.004
Kitto LJ, Henderson NC (2020). Hepatic stellate cell regulation of liver regeneration and repair. Hepatology Communications 5 (3):358-370 https://doi.org/10.1002/hep4.1628
Wake K (2006). Hepatic stellate cells: Three-dimensional structure, localization, heterogeneity and development. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences 82 (4), 155. https://doi.org/10.2183/PJAB.82.155
Weber RZ, Perron P, Rust R (2021). Astrocytes for brain repair: More than just a neuron’s sidekick. Brain Pathology 31 (5), e12999. https://doi.org/10.1111/BPA.12999
Yang T et al. (2020). Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Frontiers in Cellular Neuroscience 14. https://doi.org/10.3389/FNCEL.2020.00078
