
Physiology News Magazine
Bone loss in microgravity
That bone is lost in space is now commonly known, but this recognition was quite a surprise when human spaceflight began. What is less well known, but no less true, is that the loss concentrates on the leg bones. Is it caused by fluid shifts, simple mechanics or space food?
Features
Bone loss in microgravity
That bone is lost in space is now commonly known, but this recognition was quite a surprise when human spaceflight began. What is less well known, but no less true, is that the loss concentrates on the leg bones. Is it caused by fluid shifts, simple mechanics or space food?
Features
Jörn Rittweger
German Aerospace Center, Cologne, Germany and Manchester Metropolitan University, UK
Petra Frings-Meuthen
German Aerospace Center, Cologne, Germany
https://doi.org/10.36866/pn.92.38
Loss of density in the leg bones can amount to a reduction of one-quarter within 6 months of spaceflight (Vico et al. 2000), a magnitude and rate that seem to outweigh the bone losses of 5–10% experienced by women after menopause. A substantial risk of fracture would thus arise for long-term missions were they done without adequate countermeasures. So, how could we try to prevent that kind of bone loss, or, as the physiologist would say, what is the cause of it?
Fluid re-distribution in space
Astronauts, when afloat in space, define ‘up’ as where their head is. Fluid cannot take such arbitrary decisions, but has to move as pressure dictates. An important source of venous blood pressure arises from the tension of the vessel walls. This tension is greatest in the lower legs, thus improving the return of blood when we are upright. When we are not, including sojourns in microgravity, a good half litre of blood is pushed towards the head, and this drainage is thought to cause the notorious stork legs and the ‘puffy’ face in space. A clever school of thought had counted together the fluid redistribution and leg-accentuated bone loss in space and proposed that perfusion pressure gradients drive bone alterations. As highlighted in Charles Turner’s beautiful polemic (Turner, 1999), there even seems to be a small gain in bone mineral content (BMC) in the skull (see Fig. 2a), thus yielding a perfect match between hydrostatic pressure change and bone loss for (some of) the data gathered during a bed rest study by Adrian LeBlanc’s group (LeBlanc et al. 1990) with dual energy x-ray absorptiometry (DXA).
More recent evidence, however, contradicts this hypothesis. We have to remind ourselves that DXA only assesses the two-dimensional projection of bone mineral and soft tissues. As such, it is unable to provide a three-dimensional description. Moreover, its outcome is likely to be affected by fluid shifts, depending on the software used. A more modern approach with computed tomography demonstrates, for example, that immobilization-induced bone loss is greater at the proximal end (close to the knee, i.e. upper) than in the shaft of the human tibia (see Fig. 2b). This is found in bed rest (Rittweger et al. 2005, 2009) as well as in paraplegia (Rittweger et al. 2010), and it should not happen if bone losses were solely determined by fluid pressure changes. As to paraplegia, it is also noteworthy that passive standing of patients does not prevent bone losses (Goemaere et al. 1994), which again should not be the case were the postulated fluid pressure mechanism effective. Finally, tibial bone is also lost in unilateral lower limb suspension (ULLS), as demonstrated in Fig. 2c (Rittweger et al. 2006), which banefully erodes that postulated hypothesis. So, we probably need to employ another school of thought.
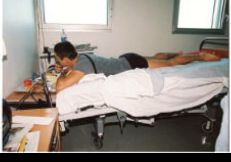
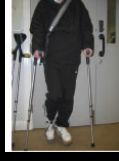
Figure 1. ESA astronaut André Kuipers when docking at the International Space Station (ISS). The cephalad fluid shifts, as well as the musculoskeletal effects of microgravity can be replicated on Earth by bed rest with –6 degrees head-down tilt. More recently, unilateral lower limb suspension has been established as a more localized model of disuse.

Microgravity on board the ISS
Astronauts on board the international space station fly in a low-Earth orbit, at a height of approximately 400 km. Although Earth’s gravity is almost completely effective at this distance, the station’s velocity of 7 km per second causes a centrifugal acceleration of an exactly equal force, cancelling-out the Earth’s pull. As a result, astronauts are afloat onboard the station, and the so-called ‘microgravity’ is only disturbed by minor imperfections of the orbit, e.g. by aerodynamic drag.
Mechanics – from single cell effects to musculoskeletal interaction
There is now ample evidence that bones adapt to mechanical stimuli (Rubin & Lanyon, 1987), although how exactly this mechano-adaptation works is unknown. Osteocytes, i.e. cells that reside within the solid phase of bone tissue, are thought to play a crucial role in it, and communication between them and osteoblasts and osteoclasts involves a symphony of paracrine signals such as RANKL, osteoprotegerin, sclerostin, DKK1 and others. Bone loss in terrestrial immobilization and in space could thus be regarded as a mechano-adaptation of bone that removes unnecessary material. This notion receives support by the way in which bone losses recover after bed rest; the accrual rate is remarkably high, and at the same time extremely accurate in anatomical terms (Rittweger & Felsenberg, 2009).
But where do the mechanical stimuli that matter to bone come from? It is true that many cells, including bone cells, are directly responsive to gravity. However, the forces caused by gravity per se are very small, e.g. 0.1 pN for an osteocyte in its aqueous environment (Cowin, 1998). As already stated, bone losses occur in the legs, but not in the arms, both in bed rest, where gravity is at work, and in space, i.e. in microgravity. It is tempting to conclude that bone losses are somehow related to the supporting function of the legs. However, as already stated, passive standing is quite ineffective for bone in paraplegic patients. On the other hand, functional electrical stimulation of paraplegic muscles can increase BMC in the local bones (Belanger et al. 2000). Is it possible that muscle contractions play a decisive role then? Mechanical engineering usually focuses on the largest loads expected for a structure. And indeed our muscles all work against short levers, and are therefore expected to generate the largest forces in the bones, not only in the arm but also in the leg (Maganaris et al. 2011). In line with this ‘muscle–bone’ hypothesis, resistive exercise during bed rest fails to maintain bone when ineffective for muscle (Rittweger et al. 2005), but proves an effective countermeasure when it does preserve muscle strength (Shackelford et al. 2004; Rittweger et al. 2010). Finally, direct evidence from a recent study of the DLR lab in Cologne demonstrates that gravitational loading of the tibia per se is insufficient to maintain bone mass, further establishing the specific importance of muscle contractions for bone (Ducos et al., in preparation). Thus, there is sufficient evidence now to put muscle–bone interaction in the first line of rationales for countermeasure development – but will this be all that there is to the story, physiologically speaking?
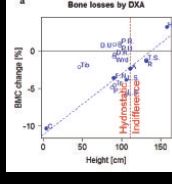

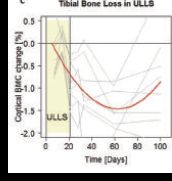
Figure 2. Some of the evidence for and against the ‘fluid pressure’ hypothesis. a, changes in bone mineral following 17 weeks of strict bed rest as assessed by dual energy absorptiometry (DXA). The original data (LeBlanc et al. 1990) from each anatomical region are plotted against the relative height within the body (Clauser et al. 1969). A strong correlation exists for the data included in Turner’s perspective note (Turner, 1999), but not for the rest of the original data (LeBlanc et al. 1990). Moreover, even for the restricted data set, the regression predicts bone losses for the hydrostatic indifference level, which speaks against hydrostatic fluid pressure as the sole mechanism. C, calcaneus; F.N., femoral neck; R, ribs; H, head. b, bone losses at different levels within the tibia as assessed by peripheral quantitative computed tomography (pQCT). This technique allows 3-dimensional assessments. The 0% and 100% sites correspond to the lower and upper tibia ends, respectively. Bone losses are substantially greater at both ends than in the shaft, both after 35-day bed rest (Rittweger et al. 2009) (red) and in paraplegic patients (Rittweger et al. 2010)(blue). Curves illustrate 3rd order polynomial fits. Again, these observations undermine the concept of hydrostatically driven mechano-adaptation. c, changes in distal tibia BMC as assessed during, and in particular following 24 days of unilateral limb suspension (Rittweger et al. 2006). Grey curves denote time courses of individual subjects, and the red curve displays a 2nd order polynomial fit.
Hormonal alterations and diet
We all know that diet matters to our bones. At least we seem to know this for calcium and vitamin D, which are probably the two mostly investigated agents in our daily diet. Of note, some people had initially thought that bone loss in space is caused by vitamin D deficiency. This proposition has now been abandoned, and dietary recommendations for astronauts are no higher than those for the terrestrial population. But what about the plethora of other nutrients that affect bone metabolism either positively (e.g. vitamin K, potassium, alkaline forming food) or negatively (e.g. high NaCl intake, acid forming food) – could any of those contribute?
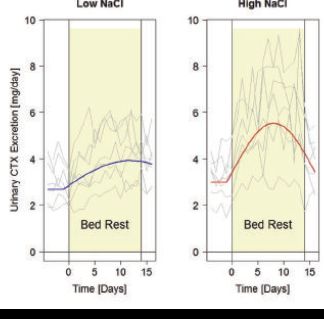
As it happens, people seem to lose some sense of taste whilst in space. Due to food conservation and to compensate for the loss of flavour, astronaut’s food items are often very salty. This could be cataclysmic, because high salt intake is likely to foster calcium excretion and bone resorption, through an acidotic shift in the milieu intérieur (Frings-Meuthen et al. 2008). Even more importantly, high salt intake can double bed rest-induced bone resorption (see Fig. 3) (Frings-Meuthen et al. 2011), and the same has been found for nitrogen losses, indicative of either impeded protein synthesis or increased degradation rate in the musculature. These detrimental effects of salt on nitrogen balance can be neutralized by a more alkaline diet (Buehlmeier et al. 2012), so that space cuisine has nowadays become an important playground for countermeasure development – and thus for physiological research!
References
Belanger M, Stein RB, Wheeler GD, Gordon T & Leduc B (2000). Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 81, 1090–1098.
Buehlmeier J, Frings-Meuthen P, Remer T, et al. (2012). Alkaline salts to counteract bone resorption and protein wasting induced by high salt intake: results of a randomized controlled trial. J Clin Endocrinol Metab 97, 4789–4797.
Clauser C, McConville J & Young J (1969). Weight, Volume, and Center of Mass of Segments of the Human Body. Aerospace Medical Research Laboratory, Aerospace Medical Division, Airforce Systems Command, Wright-Patterson Air Force Base, OH, USA.
Cowin SC (1998). On mechanosensation in bone under microgravity. Bone 22(5 Suppl), 119S–125S.
Frings-Meuthen P, Baecker N & Heer M (2008). Low-grade metabolic acidosis may be the cause of sodium chloride-induced exaggerated bone resorption. J Bone Miner Res 23, 517–524.
Frings-Meuthen P, Buehlmeier J, Baecker N, et al. (2011). High sodium chloride intake exacerbates immobilization-induced bone resorption and protein losses. J Appl Physiol 111, 537–542.
Goemaere S, van Laere M, de Neve P & Kaufman M (1994). Bone mineral status in paraplegic patients who do or do not perform standing. Osteoporos Int 4, 138–143.
LeBlanc AD, Schneider VS, Evans HJ, Engelbretson DA & Krebs JM (1990). Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5, 843–850.
Maganaris CN, Rittweger J & Narici MV (2011). Adaptive processes in human bone and tendon. In Strength and Conditioning. Biological Principles and Practical Applications, ed. Cardinale M, Newton R & Nosaka K, pp. 137–147. Wiley-Blackwell, Oxford.
Rittweger J, Beller G, Armbrecht G, et al. (2010). Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone 46, 137–147.
Rittweger J & Felsenberg D (2009). Recovery of muscle atrophy and bone loss from 90 days bed rest: Results from a one-year follow-up. Bone 44, 214–224.
Rittweger J, Frost HM, Schiessl H, et al. (2005). Muscle atrophy and bone loss after 90 days of bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36, 1019–1029.
Rittweger J, Goosey-Tolfrey VL, Cointry G & Ferretti JL (2010). Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone 47, 511–518.
Rittweger J, Simunic B, Bilancio G, et al. (2009). Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone 44, 612–618.
Rittweger J, Winwood K, Seynnes O, et al. (2006). Bone loss from the human distal tibia epiphysis during 24 days of unilateral limb suspension. J Physiol 577, 331–337.
Rubin CT & Lanyon LE (1987). Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res 5, 300–310.
Shackelford LC, LeBlanc AD, Driscoll TB, et al. (2004). Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97, 119–129.
Turner CH (1999). Site-specific skeletal effects of exercise: Importance of interstitial fluid pressure. Bone 24, 161–162.
Vico L, Collet P, Guignandon A, et al. (2000). Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355, 1607–1611.
