Physiology News Magazine
Conditions under which systemic lactate may act as a metabolic substrate for the brain
Features
Conditions under which systemic lactate may act as a metabolic substrate for the brain
Features
Angus M Brown (1) & Malcolm J W Prior (2)
1: MRC Applied Neuroscience Group, School of Biomedical Sciences
2: The Sir Peter Mansfield Magnetic Resonance Centre, School of Physics and Astronomy University of Nottingham, Nottingham, UK
https://doi.org/10.36866/pn.56.20

Ask any student what substrate the brain uses as a fuel and the answer will invariably be glucose. So ingrained is the concept of glucose as the brain’s only energy substrate that it is the central dogma of brain energy metabolism and can be summarised as follows: the brain contains insignificant energy reserves, and is thus entirely dependent on the blood to deliver a constant, uninterrupted supply of glucose in excess of the brain’s demand.
The reasons for this universally accepted assumption of glucose as the sole substrate for the brain include:
• complex homeostatic mechanisms have evolved that ensure a stable concentration of glucose in the blood ([glucose]blood) of ~ 6 mM, thus the delivery of glucose to the brain is constant;
• non-glucose substrates in the blood are converted to glucose by the liver and kidneys implying glucose is the preferred substrate;
• the brain contains neurones which respond to decreases in [glucose]blood and promote compensatory mechanisms designed to elevate [glucose]blood;
• the a-v difference in [glucose]blood is always positive, implying that the brain takes up glucose;
• NMR studies have shown that labelled glucose in the blood ends up as labelled glycolytic and oxidative intermediaries in the brain;
• insulin overdose which causes systemic hypoglycaemia and results in decreased delivery of glucose to the brain, results in deficits in brain function.
These factors appear to present a cast-iron case for glucose as the sole energy substrate used by the brain. However, there are certain circumstances where glucose is not the sole substrate used by the brain. In neonatal mammals the fatty acids derived from mothers’ milk provide about half of the energy substrate metabolised by the brain, and adult brain can survive on ketone bodies derived from fatty acids or pyruvate oxidation. It should be stated quite clearly at this point that in vitro brain tissue preparations can survive for extended periods of time on a host of non-glucose substrates (including the sugars mannose and fructose, the monocarboxylates lactate and pyruvate, and the ketone bodies acetoacetate and β-hydroxybutyrate), but in these preparations the blood brain barrier (BBB) is circumvented and substrates in the perfusate have unlimited access to tissue. The topic of this review is the viability of lactate in the systemic circulation supporting brain function.
Lactate infusion improves cognitive function during systemic hypoglycaemia
An intriguing possibility that has recently been proposed is that under certain pathological conditions systemic lactate can support brain function in adults. This concept flies in the face of current convention, as it is widely assumed that in adults lactate has limited access to the brain due to the selective permeability of the BBB. Using human volunteers, Stephanie Amiel’s group studied the ability of exogenously applied lactate delivered to the systemic circulation to support brain function when [glucose]blood had been rendered hypoglycaemic by the infusion of insulin (Maran et al. 1994). In these carefully controlled experiments when [glucose]blood was driven to hypoglycaemic levels (~2.5 mM: < 4 mM glucose is considered hypoglycaemic) infusion of lactate, leading to an eventual [lactate]blood concentration of 3.5 mM, resulted in an improvement of cognitive function when compared to volunteers who were deprived lactate infusion, implying that lactate can freely pass into the brain and be oxidatively metabolised. In addition, the counter-regulatory responses to systemic hypoglycaemia were substantially diminished. Under normal physiological conditions the a-v difference of lactate is negative, i.e. the brain produces lactate which then passes into the venous circulation. At physiological pH (pH 7.4) lactate is 99% ionised, and is co-transported with H+ ions by monocarboxylate transporters. The transport is freely reversible with equilibrium reached when:
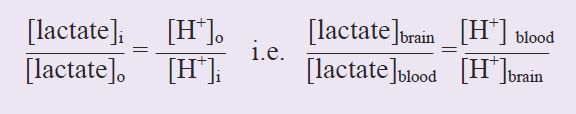
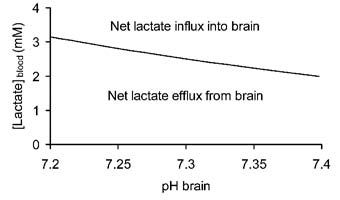
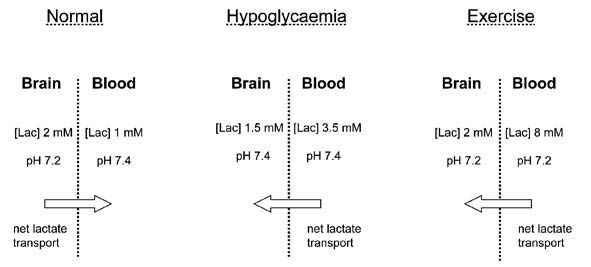
Under physiological conditions blood pH is 7.4 ([H+] = 40 nM) and brain pH is 7.2 ([H+] = 63 nM), the [lactate]brain is ~2 mM and the [lactate]blood is ~1 mM (Abi-Saab et al. 2002). Inserting these values into the equation demonstrates that the system is not in equilibrium, resulting in net efflux of lactate out of the brain. Assuming that all the parameters except the [lactate]blood remain constant, [lactate]blood must increase above 3.2 mM in order for net lactate transport into the brain to occur, i.e. [lactate]blood must reach a critical threshold determined by the pH gradient as well as the lactate gradient before net lactate influx into the brain occurs. This is illustrated in Fig. 1, where the [lactate]blood required to reverse transport of lactate into the brain is plotted against brain pH, with blood pH constant at 7.4, and[lactate]brain constant at 2 mM. It can be seen that as the brain becomes more alkaline, the [lactate]blood required to promote net reverse transport decreases. It has been shown by several groups that hypoglycaemia results in an alkalisation of the brain, such that brain pH ~ blood pH (Fig. 2), implying that all that is required for net lactate transport into the brain is for [lactate]blood to exceed [lactate]brain. Under hypoglycaemic conditions it is likely that the [lactate]brain stays constant or decreases, further lowering the [lactate]blood required to drive net lactate transport into the brain. Thus the response of the brain to hypoglycaemia lowers the [lactate]blood threshold at which net movement of lactate into the brain occurs.
Exercise-induced hypoglycaemia and elevated plasma lactate
Experiments under more physiological conditions support the hypothesis that systemic lactate can support brain function. During heavy exercise [glucose]blood can fall to levels considered hypoglycaemic (~2.5 mM), without producing the warning signs of systemic hypoglycaemia that a non-exercising individual would display (Felig et al. 1982). This is almost certainly correlated with the fact that [lactate]blood can increase to 8 mM during extreme exercise. Under these conditions the a-v difference in lactate reverses as the brain takes up lactate, presumably for oxidative metabolism to fuel brain function (Ide et al. 2000). As is the case with insulin-induced hypoglycaemia the response to exercise (i.e. acidification of the blood) lowers the [lactate]blood threshold at which lactate will enter the brain. These experiments are rather confused by the fact that there is still considerable glucose present in the blood, and it thus appears that the brain survives on a combination of glucose and lactate. Can it be a coincidence that the deficit in [glucose]blood during exercise compared to baseline levels (6 mM – 2.5 mM =3.5 mM) is matched by an equivalent increase in [lactate]blood (8 mM – 1 mM = 7 mM ~ 3.5 mM glucose)?
Of considerable interest is the fact that during exercise-induced hypoglycaemia there is no activation of counter-regulatory mechanisms suggesting that the glucose-sensing neurones in the brain are not activated due to the increased [lactate]blood. This has been verified in a separate study in rats subjected to systemic hypoglycaemia (Borg et al. 2003). Animals in which lactate was infused into the ventromedial hypothalamus exhibited a reduction of 80 – 85% of the counter-regulatory hormonal response, implying that these glucose-sensitive cells sense glucose through glycolysis, as occurs in pancreatic β cells. However, in this study it was argued that the lactate was generated within the brain parenchyma, rather than transported across the BBB.
What is the fate of glucose and lactate once they have entered the brain? It is widely agreed that astrocytes, which comprise 50% of the brain volume, are mainly glycolytic, taking up glucose and converting it to lactate which is subsequently released into the extracellular space. Neurones, on the other hand, are clearly oxidative and there is accumulating evidence that they can take up and oxidatively metabolise astrocyte-derived lactate. Is it possible that there is cellular compartmentalisation of the substrates available to the brain during exercise, and that the systemic glucose is used by astrocytes, whereas the muscle-derived lactate is used to fuel neurones?
The data presented in this article to support the hypothesis that systemic lactate is transported across the BBB and metabolised by the brain, although convincing, are entirely circumstantial.
The extensive magnetic resonance facilities at the University of Nottingham offer an ideal environment in which to address some of the key points raised in this article, and experiments are currently underway in which NMR spectroscopic detection of metabolites derived from systemically injected labelled lactate will determine conditions under which systemic lactate crosses the BBB and is metabolised.
References
Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ & Sherwin RS (2002). Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J Cereb Blood Flow Metab 22, 271-279.
Borg MA, Tamborlane WV, Shulman GI & Sherwin RS (2003). Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52, 663-666.
Felig P, Cherif A, Minagawa A & Wahren J (1982). Hypoglycemia during prolonged exercise in normal men. N Engl J Med 306, 895-900.
Ide K, Schmalbruch IK, Quistorff B, Horn A & Secher NH (2000). Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol 522, 159-164.
Maran A, Cranston I, Lomas J, Macdonald I & Amiel SA (1994). Protection by lactate of cerebral function during hypoglycaemia. Lancet 343, 16-20.