
Physiology News Magazine
Developing insights into Purkinje cell maturation
Recent evidence reveals a temporal correspondence between the maturation of spike output properties and cell structure during postnatal Purkinje cell development, which further parallels the development of anatomical, synaptic and behavioural indices of cerebellar maturation
Features
Membership
Developing insights into Purkinje cell maturation
Recent evidence reveals a temporal correspondence between the maturation of spike output properties and cell structure during postnatal Purkinje cell development, which further parallels the development of anatomical, synaptic and behavioural indices of cerebellar maturation
Features
Membership
Bruce E McKay & Ray W Turner
Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta, Canada
https://doi.org/10.36866/pn.63.24

The cerebellum coordinates fine motor movements, underlies the adaptation of ocular responses, and supports the learning of some conditioned behaviours (Ito, 1984). However, in the rat and mouse, these behaviours, and the anatomy and physiology that enable these functions, are either absent in the altricial neonate, or present in an immature form (Altman & Bayer, 1997).
At birth the cerebellum is limited to a pair of small masses overlying the fourth ventricle, with Purkinje cells arranged in clusters and granule cells present in an external layer of the cortex. Neuronal morphology is immature, synaptic innervation is rudimentary, and the repertoire of cerebellar-dependent behaviours is limited (Altman & Bayer, 1997). By the third to fourth postnatal weeks the anatomy, synaptic connectivity and neuronal morphology, as well as behavioural indices of cerebellar function, all reach adult levels (Altman & Bayer, 1997). The development of these characteristics is known with precise temporal resolution. However, the development of intrinsic neuronal properties, such as spike output patterns, and any relationship to the development of cell morphology, had not been determined with high temporal resolution for any cerebellar cell type.
To this end we studied the structural and functional development of Purkinje cells, a cell type of central importance to overall cerebellar function, from P0 through P90 using whole-cell patch clamp recordings and Neurobiotin cell fills in the in vitro slice preparation(McKay & Turner, 2005). We found that both morphological and electrophysiological characteristics develop with a sigmoidal time course: an initial stable immature stage of minimal change from P0 to ~P9, a transition stage from >P9 to ~P18 wherein the conversion from immature to adult characteristics occurs, and finally a stable adult stage >P18 where only minor refinements are noted (McKay & Turner, 2005). For instance, during the stable immature stage Purkinje cells possess a peri-somatic dendritic configuration (e.g., P3 and P9, Fig. 1A). During the transition stage Purkinje cells retract their peri-somatic dendrites and then generate a single primary stem dendrite that rapidly increases in surface area to nearly adult levels (e.g., P12 and P18, Fig. 1A). During the stable adult stage only small morphological changes are observed (Fig. 1A and 1E).
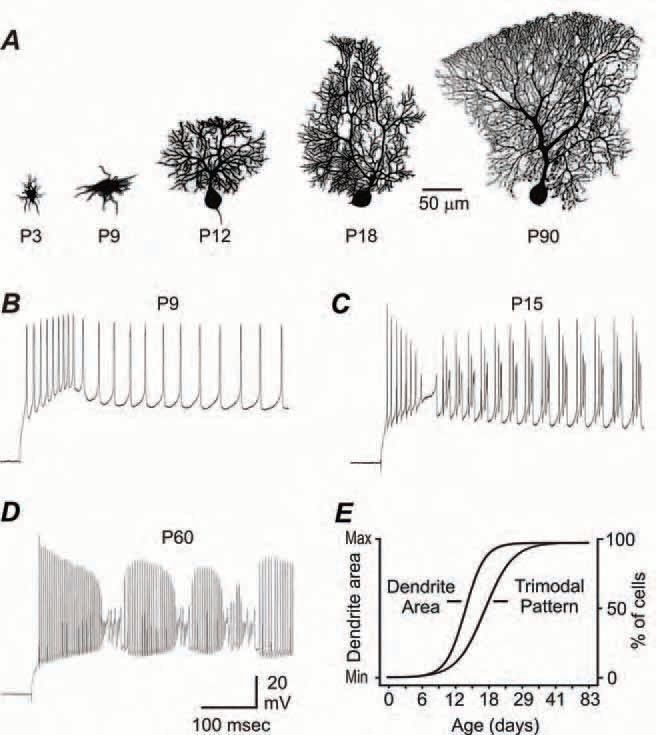
Occurring alongside morphological changes is a parallel shift in the patterns of evoked and spontaneous spike output. Throughout the stable immature stage intracellular stimulation evokes a transient low threshold burst discharge of action potentials (Fig. 1B). However, during the transition stage Purkinje cells proceed through an under-developed form of repetitive high threshold bursts (Fig. 1C), which ultimately matures to a stable adult pattern of high threshold burst output (Fig. 1D). Following a similar time course, the percentage of cells generating the trimodal (tonic, burst, quiet) pattern of spontaneous output is 0% throughout the stable immature stage and quickly increases to nearly 100% by the end of the transition stage (Fig. 1E). Co-plotting the Boltzmann fits (see McKay & Turner, 2005) of the developmental changes in dendritic surface area and the proportion of Purkinje cells generating the trimodal pattern reveals the relative postnatal time courses for these two variables. This strategy indicates that dendritic development precedes the development of trimodal output by a few days, but importantly reveals that, once initiated, these characteristics mature at similar rates.
Interestingly, the time courses of multiple aspects of cerebellar development, including the maturation of gross anatomy, synaptic innervation and behavioural indices appear to parallel the time course of development we describe for Purkinje cell structure and spike output.
For instance, one measure of gross anatomy – the cross-sectional area of the vermis – changes minimally during approximately the first postnatal week (stable immature stage), undergoes a massive expansion to adult size over the next two weeks (transition stage), and then plateaus in area (stable adult stage) (Altman & Bayer, 1997). Also following a sigmoidal time course is the development of synaptic inputs to Purkinje cells. Throughout the first postnatal week only a small number of Purkinje cell parallel fibre and basket cell synapses are detected, whereas a marked proliferation is noted during the second postnatal week with the attainment of stable adult levels by the third postnatal week (Larramendi, 1969). Further, the number of climbing fibres that synapse onto each Purkinje cell is stable at 3-4 during the first postnatal week, decreases to one climbing fibre per Purkinje cell during the second postnatal week, and remains stable at this level throughout adulthood (Scelfo & Strata, 2005). Finally, the maturation of known cerebellar-dependent behaviours, including pelvis elevation, maintaining balance on a narrow plank, mid air righting, and rearing without support, are essentially absent during the first postnatal week, improve significantly during the second and third postnatal weeks, and stabilize at adult values by the fourth postnatal week, further mirroring the development of Purkinje cell characteristics (Altman & Bayer, 1997).
Thus despite the diversity of these anatomical, synaptic, cellular and behavioural indices, the temporal progression towards adult-like character follows a similar sigmoidal time course – each characteristic begins with an initial stage of stable immaturity persisting for approximately the first postnatal week, rapidly progresses through a transition stage over the course of only approximately one week, and then persists in a stable adult stage.
The use of Boltzmann functions to analyze a range of developmental variables may prove useful in establishing a comprehensive and quantitative picture of multiple aspects of postnatal cerebellar development.
Acknowledgements
This work was funded by the Canadian Institutes for Health Research (RWT, BEM), the Alberta Heritage Foundation for Medical Research (RWT), the Killam Trust (BEM) and a Steinhauer Doctoral Award (BEM).
References
Altman J & Bayer SA (1997). Development of the Cerebellar System. CRC Press, New York.
Ito M (1984). The cerebellum and neural control. Raven, New York.
Larramendi LMH (1969). Analysis of synaptogenesis in the cerebellum of the mouse. In Neurobiology of cerebellar evolution and development. ed. Llinas R. American Medical Association, Chicago.
McKay BE & Turner RW (2005). Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol 567, 829850.
Scelfo B & Strata P (2005). Correlation between multiple climbing fibre regression and parallel fibre response development in the postnatal mouse cerebellum. Eur J Neurosci 21, 971-978.
