
Physiology News Magazine
Getting to the heart of it
The cardiovascular consequences of spaceflight
Features
Getting to the heart of it
The cardiovascular consequences of spaceflight
Features
Peter Norsk
Baylor College of Medicine, TX, USA
https://doi.org/10.36866/pn.117.40
Human spaceflight started 58 years ago with the launch of the Russian cosmonaut, Yuri Gagarin, who completed one orbit around Earth on 12 April 1961. Eight years later, 50 years ago, NASA landed the first humans, Neil Armstrong and Edwin (Buzz) Aldrin, on the lunar surface with Apollo 11. Since then, the longest human mission in space is 438 days on board the Russian Space Station, Mir, by Valery Polyakov in 1995. As of today, 574 humans have visited space. The return journey to Mars from Earth will take 1,000 days, and there are several physiological challenges to overcome in bringing humans safely back to Earth.

One is managing the shift of blood and fluid into the heart and head that occurs during weightlessness and ensuring that no harm will occur to central nervous function and the cardiovascular system. Another is maintaining adequate muscle and bone strength by efficient exercise prescriptions with specialised equipment, designed to fit into small vehicles and habitats. Another concern is maintaining an efficient immune system and supplying the crews with sufficiently nutritious and palatable food. Finally, it is a challenge to protect astronauts against the long-term effects of space radiation, particularly the effect on brain function and the cardiovascular system.
Pumping blood in a weightless environment
My space research career started in August of 1978. Before that, I had been fascinated by the Apollo program and as a high school student during the Moon landings, I knew all the details of the missions. This fostered an interest in combining my medical studies with spaceflight health issues, and in 1978 I became a student fellow in a Danish space physiological research group using a national grant to support scientists, who participated in the human spaceflight programs of the European Space Agency (ESA). We specialised in how the cardiovascular system and the associated regulation of fluid and electrolyte balance adapts to weightlessness in space.
A prominent problem was – and still is – the magnitude of the headward blood and fluid shift that occurs because of weightlessness (0 G), where no gravitationally induced hydrostatic pressure gradients exist. In 1993, we aimed to estimate the change in central venous pressure (CVP) in one astronaut before, during and a couple of hours after launch into space in the Space Shuttle to estimate the increase in cardiac preload caused by the fluid shift. In two previous Space Shuttle missions, a US team had observed – much to their and our surprise – that CVP in three astronauts decreased in space compared to in the supine body position on the ground (Buckey et al., 1996). We observed the same decrease (Foldager et al., 1996). This was surprising, because the expectation had been that the fluid shift in weightlessness would induce an increase in CVP to somewhat above that of being 1 G supine. In both cases, CVP was measured through invasive catheters fed into the superior vena cava through a cubital vein either with a fluid-filled system connected to a transducer outside the body (Buckey et al., 1996) or with a transducer at the catheter tip (Foldager et al., 1996).
Shortly thereafter, we (Videbaek and Norsk, 1997) repeated the measurements in eight subjects during 20 seconds of 0 G during parabolic flights (Fig. 1). Buckey et al. (1996) had also observed in their three astronauts that even though CVP decreased in space, left ventricular end diastolic volume as estimated by echocardiography increased; a paradoxical result. However, our parabolic flight data revealed the same, but we also measured oesophageal pressure through a balloon-tipped air-filled tube positioned via the nasal cavity, down the oesophagus to lodge behind the heart. Simultaneously with the decrease in CVP, we observed an even greater decrease in oesophageal pressure indicating that 0 G expands the thoracic cage compared to being 1 G supine. This mechanical expansion dilates the heart and increases transmural CVP. Cardiac preload therefore increases in weightlessness as a combination of an expansion of the thorax and the head-ward fluid shift (Videbaek and Norsk, 1997).

According to Starling’s law of the heart (Patterson and Starling, 1914) an increase in cardiac preload induces an increase in stroke volume and thus cardiac output, a phenomenon that also occurs in space. We observed in an investigation on the Space Shuttle in 2003 an increase in stroke volume and cardiac output of 22% (Norsk et al., 2006) a week into the flight. Later on the International Space Station, we found even more pronounced increases of 35 – 41% (Norsk et al., 2015) between three and six months of flight. Thus, stroke volume and cardiac output increase more during long-duration flights than short. The same foreign gas rebreathing technique for both space missions for estimating cardiac output was used (Clemensen et al., 1994; Gabrielsen et al., 2002). The reason for the more pronounced increase in stroke volume and cardiac output during longer missions versus shorter is not clear at present but could be because of the efficient cardiovascular effects of the physical exercise on the International Space Station (Hughson et al., 2012).
At the same time as cardiac output is increased by 0 G in space, systolic, diastolic and mean arterial pressures decrease by some 10 mm Hg (Shykoff et al., 1996; Baevsky et al., 2007; Norsk et al., 2015).
Thus, systemic vascular resistance decreases (calculated as mean arterial pressure divided by cardiac output) (Shykoff et al., 1996; Norsk et al., 2015). The mechanism for the decrease in systemic vascular resistance, which is indicative of peripheral arterial vasodilation, is currently unknown, because sympathetic nervous activity is maintained in space at the same level as being seated upright on the ground (Norsk et al., 2015; Fig. 2). A suppression of the efferent sympathetic nervous activity is therefore not the mechanism for the decrease in systemic vascular resistance.
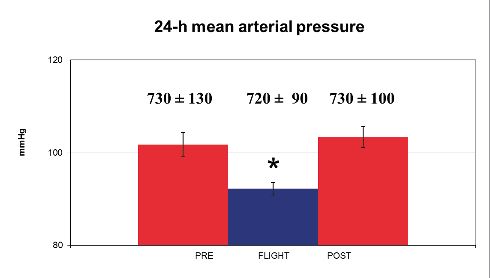
To identify the mechanisms of the blood pressure reduction and peripheral vasodilatation in space is one of the challenges for the future.
Spaceflight-associated neuro-ocular syndrome (SANS)
More than 10 years ago, astronauts started to report occurrences of vision changes in space. They were mostly of refractory character and corrected for by adjustable glasses (Stenger et al., 2017). NASA flight surgeons started to monitor other ocular variables such as retinal changes by fundoscopy, which in some cases indicated disc oedema. By using optical coherence tomography (OCT) and ultrasound imaging, more details have later been obtained, and choroidal folds, cotton wool spots, increases in optic nerve sheath diameter and globe flattening have also been detected (Mader et al., 2011; Stenger et al., 2017). Collectively, one or more of these manifestations constitute what is called SANS. Recent data have revealed that disc oedema is preceded by gradual increases in retinal thickness. Laurie et al. (2019) were able to induce some of these changes during strict, 30 days of six degrees head-down tilted bed rest, which indicates that the shift of blood and fluid to the head is a pivotal mechanism. It appears that some astronauts are more sensitive to SANS than others, indicating that there maybe a genetic disposition to this syndrome (Smith and Zwart, 2018).
SANS is one of the highest prioritised health risks of long-duration spaceflight, and much research is initiated by the space agencies to understand the details of the mechanisms of the syndrome and how to counteract it during, for example, a three-year mission to Mars.
In addition to SANS, concerns also exist regarding possible impacts of spaceflight on not only the ocular but also the central nervous system. Structural changes by magnetic resonance imaging of the brain in the days following landing from the International Space Station have been detected (Roberts et al., 2018). Whether they have any clinical health or functional impacts are unknown at present.
Cardiovascular remodelling in spaceflight
Results of ground-based rodent studies have indicated that changing the pressure distribution by shifting blood and fluid to or away from the head by body tilts induces changes in the structure of the arterial walls (Zhang, 2013). Thickening of arterial walls is also evident in hypertension, for example (Mulvany, 2012). Data from two studies on the International Space Station indicate that the carotid artery increases in stiffness during and immediately following spaceflight (Hughson et al., 2016; Arbeille et al., 2017). In one astronaut with almost one year in space on the International Space Station (340 days), carotid wall thickness increased as well as biomarkers indicating increased inflammation and oxidative stress in the cardiovascular system (Garrett-Bakelman FE et al., 2019). Thus, there are some indications that the carotid arterial wall structure may change during long-duration spaceflight. The clinical implications of these observations are, however, currently unknown.
Other physiological effects
In 2011, I joined NASA as a contractor to lead the physiological research in the Human Research Program. At that time, I had been mostly involved in cardiovascular and fluid volume regulation research concerning spaceflight. Thus, I had to understand the status of other physiological areas concerning effects of long-duration flights.
Apart from the cardiovascular system, the main areas of concern are the ocular, central nervous, sensorimotor, immune and musculoskeletal systems and to determine the most efficient nutritional composition of the food systems to maintain astronaut health and performance during long-duration missions. Regarding the musculoskeletal system, maintaining muscle strength for performance of the required mission tasks is important, as is maintaining sufficient bone strength to avoid an increased risk of fracture due to bone demineralisation. The key countermeasure against these effects is regular resistance exercise. The future challenge is to develop an exercise device that can be accommodated in a small space vehicle or a habitat and still be able to fulfill the musculoskeletal and aerobic requirements (Ploutz-Snyder et al., 2014). SANS is of particular concern, as well as whether brain function is affected (Mader et al., 2011; Stenger et al., 2017).
We know how weightlessness affects the sensorimotor system, and that all astronauts experience balance problems immediately after landing (Mulavara et al., 2018). This is of concern after landing on Mars, where the key question is, how long after landing will it take the astronauts to recover and be able to perform the required mission tasks? The immune system is attenuated by spaceflight, leading in some cases to viral reactivations and rashes, and major efforts are directed towards a host of protective interventions, mainly to decrease operational stress and supply foods with antioxidants (Crucian et al., 2018). Finally, defining the optimal food composition as well as identifying which food items are most applicable for physiological health protection is a challenge (Smith and Zwart, 2018).
Future plans for deep space missions
NASA is currently planning for future human deep space missions beyond the van Allen Belts with the so-called Gateway project. The plans concern regular human missions to a small habitat orbiting the Moon. The idea is to have a crew of four visit the habitat for some 45 – 60 days on an annual basis with gradual increases in durations as a human test bed for understanding the impacts of the deep space environment on human physiology. The plans now also include landing astronauts from the Gateway habitat to the surface of the Moon, with the first mission to land a female astronaut in 2024.
It is of high priority to understand the combined effects of weightlessness and deep space radiation on oxidative stress and cellular damage, and how this impacts health and performance. Currently, only 24 astronauts, as part of the Apollo program (1968 – 1972), have been in deep space and only for a maximum of 12 days. Therefore, very little is known regarding the physiological effects of long-duration exposures to the deep space environment.
Conclusion
Surprises and paradoxes concerning how the cardiovascular system adapts to spaceflight defy explanation, for example mean arterial pressure and systemic vascular resistance decrease during months of spaceflight despite an unchanged efferent sympathetic nervous tone. For future deep space missions, to the Moon in the 2020s and to Mars in the 2030s, physiologists are most concerned about the combined effects of weightlessness and space radiation on the central nervous, ocular, cardiovascular, sensorimotor and immune systems, and these will be the focus of intense research in the coming years.
References
Arbeille P et al. (2017). Carotid and femoral arterial wall distensibility during long-duration spaceflight. Aerospace Medicine and Human Performance 88, 924 – 929. DOI: 10.3357/AMHP.4884.2017
Baevsky RM et al. (2007). Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. Journal of Applied Physiology 103, 156 – 161. DOI: 10.1152/japplphysiol.00137.2007
Buckey JC Jr et al. (1996). Central venous pressure in space. Journal of Applied Physiology 81, 19 – 25. DOI: 10.1152/jappl.1996.81.1.19
Clemensen P et al. (1994). A modified photo- and magnetoacoustic multigas analyzer applied in gas exchange measurements. Journal of Applied Physiology 76, 2832 – 2839. DOI: 10.1152/jappl.1994.76.6.2832
Crucian BE et al. (2018). Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Frontiers in Immunology 9, 1437. DOI: 10.3389/fimmu.2018.01437
Foldager N et al. (1996). Central venous pressure in humans during microgravity. Journal of Applied Physiology 81, 408 – 412. DOI: 10.1152/jappl.1996.81.1.408
Gabrielsen A et al. (2002). Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clinical Science (London), 102, 247 – 252. DOI: 10.1042/cs1020247
Garrett-Bakelman FE et al. (2019) The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 364 (144), 1 – 20. DOI: 10.1126/science.aau8650
Hughson RL et al. (2012). Cardiovascular regulation during long-duration spaceflights to the International Space Station. Journal of Applied Physiology 112, 719 – 727. DOI: 10.1152/japplphysiol.01196.2011
Hughson RL et al. (2016). Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. American Journal of Physiology 310, H628 – H638. DOI: 10.1152/ajpheart.00802.2015
Laurie SS et al. (2019). Optic disc edema after 30 days of strict head-down tilt bed rest. Ophthalmology 126, 467 – 468. DOI:10.1016/j.ophtha.2018.09.042
Mader TH et al. (2011). Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058 – 2069. DOI: 10.1016/j.ophtha.2011.06.021
Mulavara AP et al. (2018). Physiological and functional alterations after spaceflight and bed rest. Medicine and Science in Sports and Exercise 50, 1961 – 1980. DOI: 10.1249/MSS.0000000000001615
Mulvany MJ (2012). Small artery remodelling in hypertension. Basic and Clinical Pharmacology and Toxicology 110, 49 – 55. DOI: 10.1111/j.1742-7843.2011.00758.x
Norsk P et al. (2006). Vasorelaxation in space. Hypertension 47, 69 – 73. DOI: 10.1113/jphysiol.2014.284869
Norsk P et al. (2015). Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. The Journal of Physiology 593, 573 – 584. DOI: 10.1113/jphysiol.2014.284869
Patterson SW, Starling EH (1914). On the mechanical factors which determine the output of the ventricles. The Journal of Physiology 48, 357 – 379. DOI: 10.1113/jphysiol.1914.sp001669
Ploutz-Snyder LL et al. (2014). Integrated resistance and aerobic exercise protects fitness during bed rest. Medicine and Science in Sports and Exercise 46, 358 – 368. DOI: 10.1249/MSS.0b013e3182a62f85
Roberts DR et al. (2018). Effects of spaceflight on astronaut brain structure. The New England Journal of Medicine 378, 582 – 583. DOI: 10.1056/NEJMoa1705129
Shykoff BE et al. (1996). Cardiovascular response to submaximal exercise in sustained microgravity. Journal of Applied Physiology 81, 26 – 32. DOI: 10.1152/jappl.1996.81.1.26
Smith SM, Zwart SR (2018). Spaceflight-related ocular changes: the potential role of genetics, and the potential of B vitamins as a countermeasure. Current Opinion in Clinical Nutrition and Metabolic Care 21, 481 – 488. DOI: 10.1097/MCO.0000000000000510
Stenger MB et al. (2017). Evidence Report: Risk of Spaceflight Associated Neuro-ocular Syndrome (SANS). [Online] NASA https://humanresearchroadmap.nasa. gov/evidence/reports/SANS.pdf
Videbaek R, Norsk P (1997). Atrial distension in humans during microgravity induced by parabolic flights. Journal of Applied Physiology 83, 1862 – 1866. DOI: 10.1152/jappl.1997.83.6.1862
Zhang LF (2013). Region-specific vascular remodeling and its prevention by artificial gravity in weightless environment. European Journal of Applied Physiology 113, 2873 – 2895. DOI: 10.1007/s00421-013-2597-8
