Physiology News Magazine
Lab profile: The Centre for Renal Tubular Physiology, University College London
Membership
Lab profile: The Centre for Renal Tubular Physiology, University College London
Membership
Stephen Walsh, University College London, UK
https://doi.org/10.36866/pn.121.42
The renal physiology laboratory was set up at the Royal Free Campus of University College London (UCL) by Professor Robert Unwin in 2001. Although Professor Unwin was trained in micropuncture at Yale, and he published widely in animal models of disease, his interest was always in patients.
Now I head the centre, and while we do some animal work to unpick the underlying fundamental physiological processes, our focus is really on humans and specifically human renal tubular physiology. Our special interest is in disorders of sodium transport, blood pressure homeostasis, and the distal convoluted tubule (DCT).
The bedrock of the centre is the UCL Tubular Clinic, where we see patients with very rare renal tubular disorders, both inherited and acquired. We look after the largest cohorts of patients with Gitelman and Bartter syndrome in the UK, as well as large cohorts of patients with Gordon syndrome, distal renal tubular acidosis, the renal Fanconi syndrome, and other rarer electrolyte and acid–base disorders. We get referrals from all over the UK, importantly including our sister clinic at Great Ormond Street Hospital, as well as frequent calls for remote advice. We offer specialist diagnostics (specialist biochemistry, genetics and physiological testing) and therapeutics (including specialist supplements and immunomodulatory treatments).
Renal tubular physiology is also very important to consider in patients who develop either kidney stones or hypertension. We have a specialist metabolic stone clinic to diagnose rare disease causes of kidney stone formation. We also set up a Complex Hypertension Clinic, where we screen patients with unusual or treatment-resistant hypertension for rare secondary causes of hypertension, and for evidence of poor patient compliance (i.e. flushing their pills down the drain!).
The rare disease patients also contribute to research; we conduct genetic studies for novel gene discovery (EAST syndrome, for example, was discovered here), carry out imaging studies of the hearts of Gitelman and Gordon syndrome patients and measure the urinary excretion of salts and exosomes in these patients when they are exposed to diuretics and other pharmacological agents. This is work that is being done by one of our clinical research training fellows, Dr Beth Wan.
This unusually dense collection of very rare human disease means that we have access to both patients and human biosamples, which have allowed Dr Keith Siew, a Sir Henry Wellcome Postdoctoral Fellow in the Centre training in micropuncture and imaging, to translate his findings from animal models to humans.
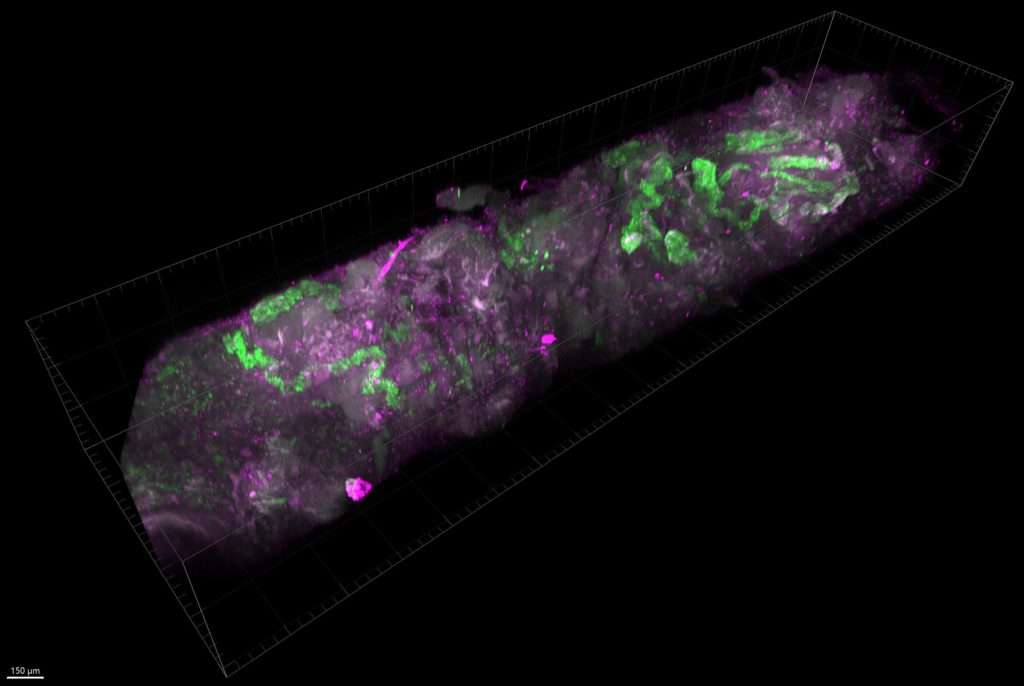
We have advanced imaging facilities and use modern optical clearing techniques to render human kidney tissue transparent (the methodology we use is called SHIELD). We then probe them with oligonucleotides, antibodies and/or stains and image them, using light sheet microscopy in 3D. We are developing this for rare disease basic research, comparative physiology, and new human diagnostic techniques, along with our consultant histopathologist, Dr Lauren Heptinstall, who is one of the researchers in the centre.
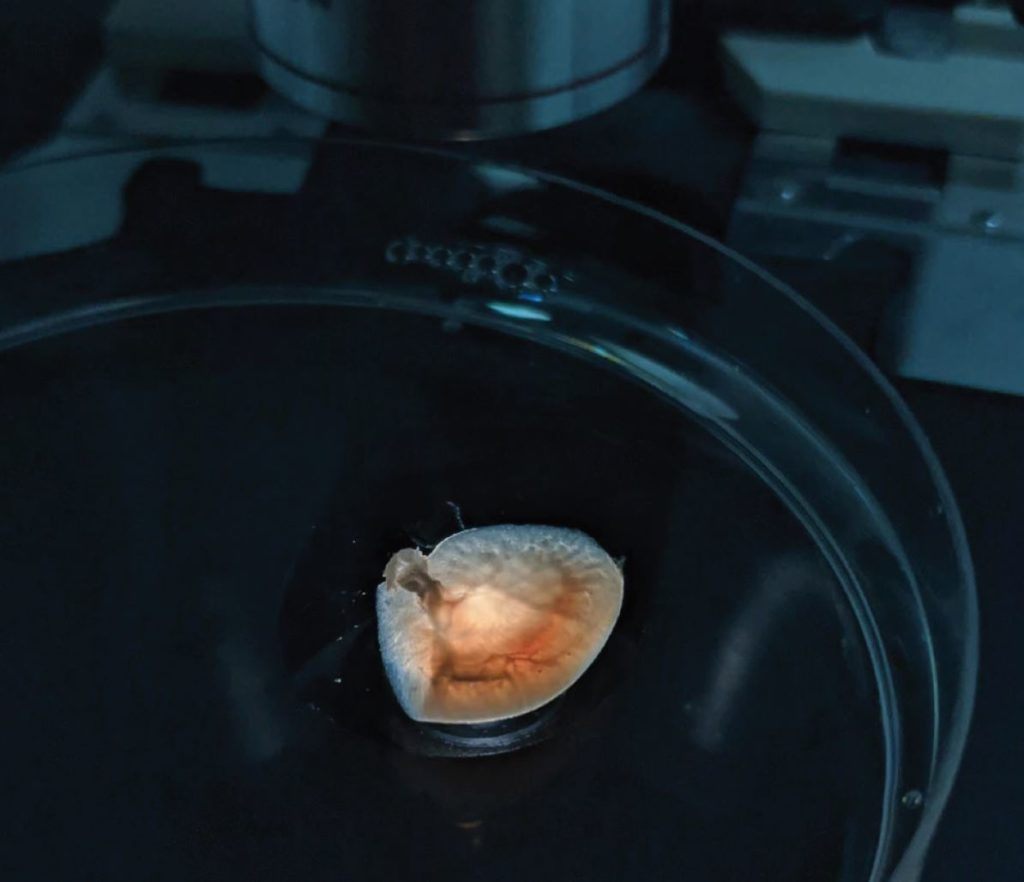
The centre was well known for the difficult micropuncture and microperfusion techniques that were pioneered at Yale. Anatomical limitations mean that distal nephron physiology is almost impossible to study using micropuncture in mice, and although humans are the right size for this, we have not yet managed to convince an ethics committee to let us try.
The clumsily named organ-on-a-chip technologies offer a way out of this ethical conundrum. We have immortalised human DCT cells and are able to culture them in 3D tubular cultures with directional luminal flow. Furthermore, we have been able to isolate and culture DCT cells from rather large volumes of human urine. Growing 3D cultures of primary human DCT cells is the next step and offers exciting new possibilities for physiology and diagnostics. This work forms part of the training for Eunice Zhong, one of our PhD students.
The centre has full cell biology, proteomic and bioinformatic capabilities and is rapidly developing the hardware and computer scientists to analyse the complex and dense data generated by these new techniques.
The geeky tech obsession that permeates the centre carries over into the lab meetings, which are conducted on a number of fashionable web-based apps (depending on the whims of Drs Walsh and Siew), in addition to the standard video conferencing apps so prevalent in COVID-19 times.