Physiology News Magazine
Octopamine is not just for arthropods
Up till recently octopamine appeared to be a purely arthropod neurotransmitter. Here Chris Elliott and Ágnes Vehovszky demonstrate that it also occurs in the Phylum Mollusca, and may occur elsewhere.
Features
Octopamine is not just for arthropods
Up till recently octopamine appeared to be a purely arthropod neurotransmitter. Here Chris Elliott and Ágnes Vehovszky demonstrate that it also occurs in the Phylum Mollusca, and may occur elsewhere.
Features
Chris Elliott
Department of Biology, University of York
Ágnes Vehovszky
Balaton Limnological Institute of the Hungarian Academy of Sciences
https://doi.org/10.36866/pn.45.17

Octopamine has been well documented as a transmitter, modulator and hormone in both crustacea and insects, (for review see Roeder, 1999), and it has provided a target for the development of at least one commercial insecticide (Amitraz, NOR-AM Chemical Company, Delaware, USA). However, we have now shown that octopamine is a major transmitter and modulator in a different phylum, the molluscs, where it plays a major role in feeding.
The octopamine antagonists known to be effective in insects block the normal feeding response of intact snails (Lymnaea stagnalis), with the most effective antagonists, phentolamine and epinastine, having the strongest effect. One possible explanation for this observation would be the presence of octopaminergic cells in the feeding system and this we have been able to demonstrate.
Octopamine as a transmitter in the buccal ganglia
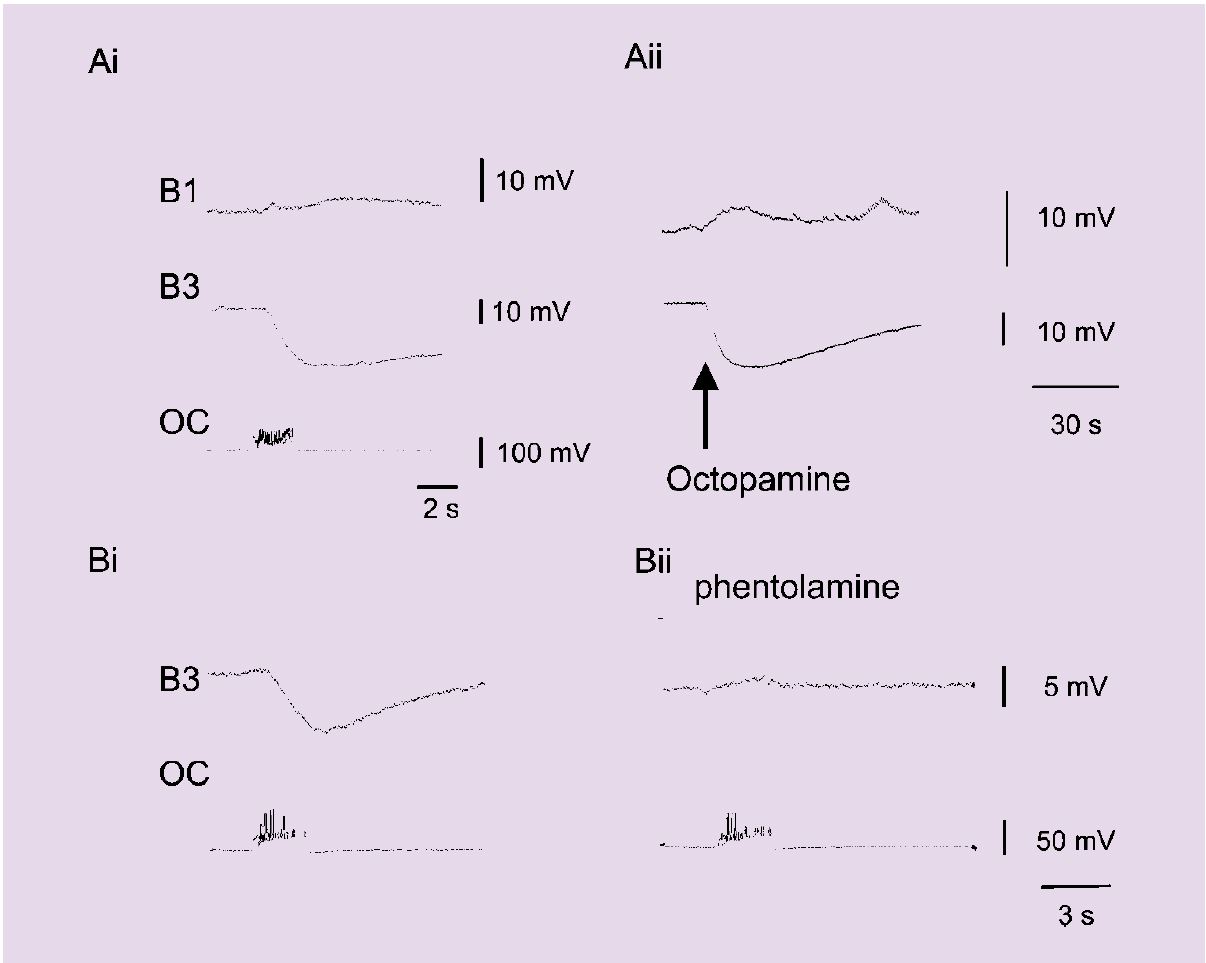
HPLC shows that the buccal ganglia, which contain about 400 cells and control feeding, have the highest concentration of octopamine, which is contained in just 3 octopamine-immunoreactive neurons (Elekes et al, 1996; Hiripi et al, 1998). These interneurons are homologous and arborise solely within the buccal ganglia (Vehovszky et al, 1998). Electrophysiological recording shows that these OC interneurons make synapses with all the known members of the feeding network. Detailed analysis of the inhibitory synapse from the OC interneuron to the B3 motoneuron shows that the connection is very effectively blocked by the octopamine antagonists phentolamine and epinastine. These also block the hyperpolarising response of the B3 motoneuron to application of octopamine from a small pipette (Figure 1). Together with saturating binding of octopamine, these observations demonstrate that octopamine is acting as a transmitter in this system.
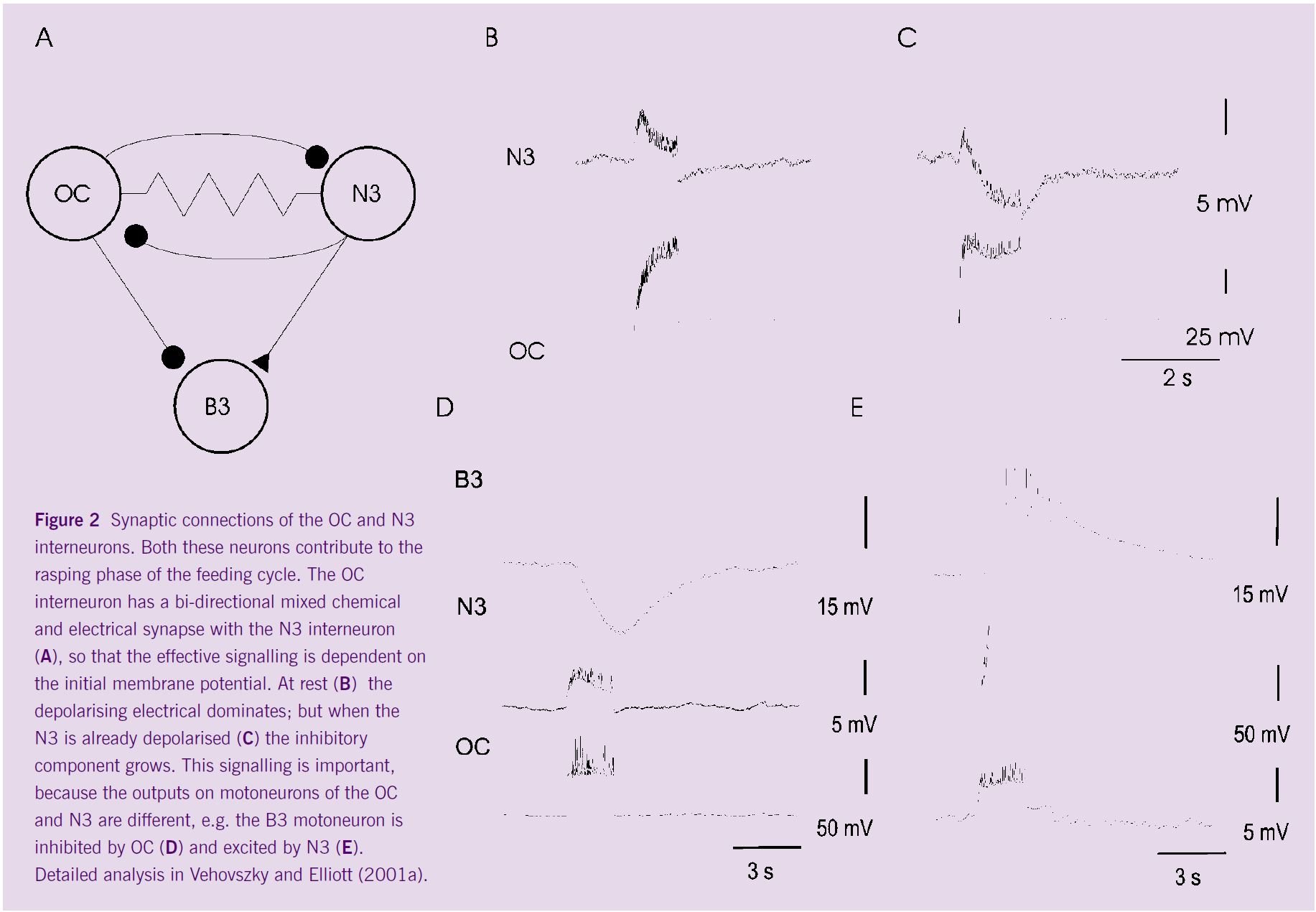
Many of the octopaminergic synapses are biphasic. Among these are the OC → N3 interneuron synapses where the inhibitory octopaminergic chemical synapse is combined with a faster electrical synapse (Figure 2). This means that the effect of the synapse is very dependent on the initial membrane potential of the postsynaptic neuron. At rest, the membrane potential is close to the reversal potential of the chemical synapse, so the dominant effect of the synapse is excitatory. If however, the N3 is already active, the electrical connection is much less effective, and the inhibitory component dominates. This part of the network is further complicated by the fact that the N3 interneuron makes a similar, non-octopaminergic synapse back onto the OC interneuron with both inhibitory chemical and electrical components (Figure 2). Similar complex synaptic arrangements have been described in other central pattern generators, including the stomatogastric ganglia of crustacea (Eisen and Marder, 1984; Evans et al, 1999) where they provide a means of tuning the pattern. This would be important in our Lymnaea feeding system too, because the OC and N3 interneurons have opposite effects on the motoneurons, including the B3 neuron (Figure 2).
Octopaminergic modulation of feeding
About one third of isolated Lymnaea CNS preparations produce a rhythmic pattern similar to the sequence of activity seen during feeding in the intact snail. This pattern, know as fictive feeding, is modulated by stimulating the OC interneuron. Initial experiments showed that stimulating the OC interneuron during fictive feeding stabilised the feeding rate, so that slow rhythms were accelerated and fast ones slowed down (Vehovszky and Elliott, 2000). However, examination of the feeding pattern shows that the pattern is also reconfigured. The changes include removal of the B3 motoneuron from patterns in which the OC interneuron is active and shifts in the timing of the rhythmic pattern (Figure 3A) (Vehovszky and Elliott, 2001a).
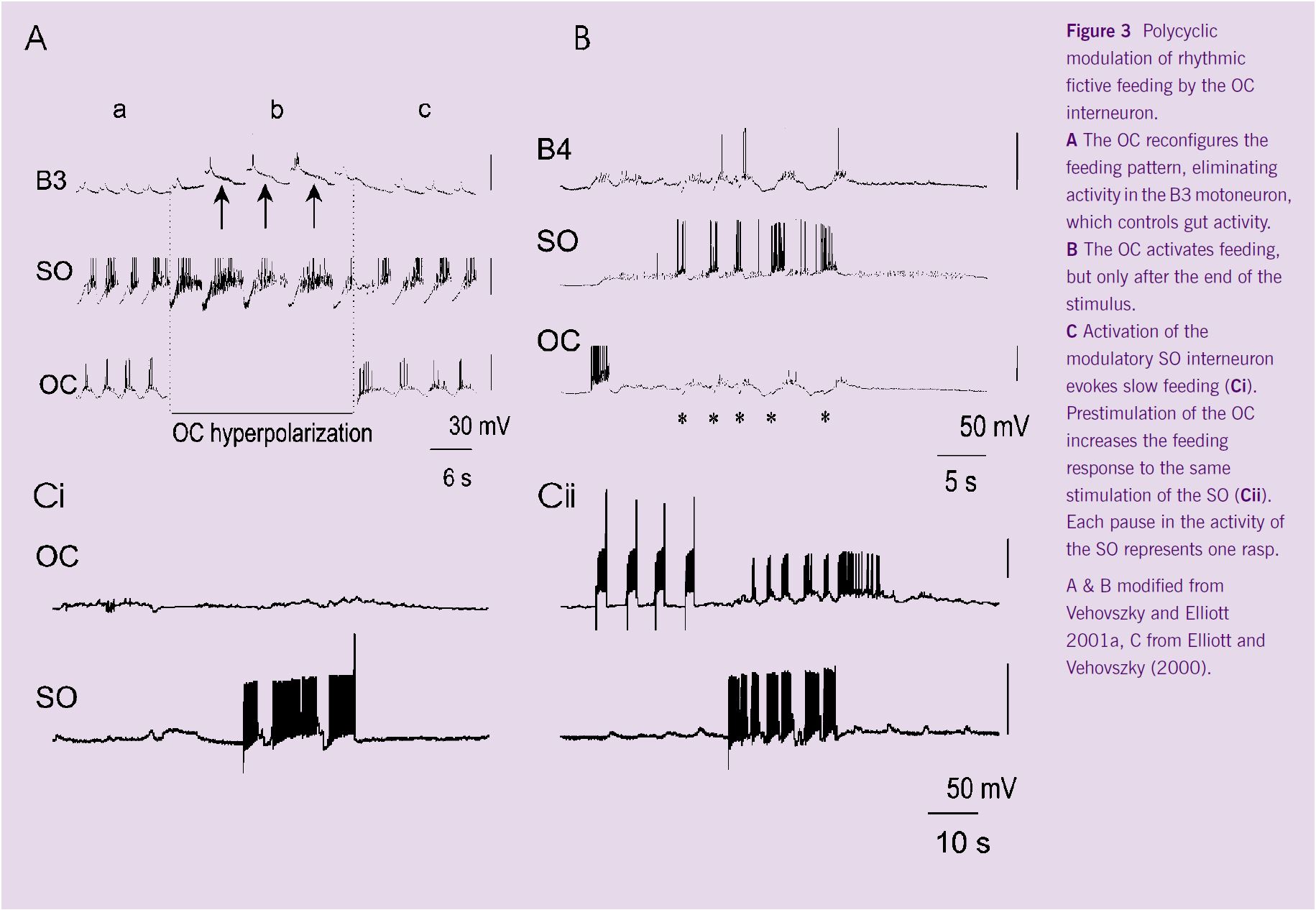
Subsequent experiments in quiescent preparations showed that stimulating the OC itself could elicit fictive feeding. Unlike all previous neurons described in molluscan feeding, the response to the OC interneuron did not occur during the period of stimulation, but 3-4s later. Furthermore, the pattern may continue for many feeding cycles (Figure 3B). This effect can be partially explained by the biphasic synapses made between the OC interneuron and the premotor interneurons (SO, N1; Vehovszky and Elliott 2001a).
In addition to the ability of the OC interneurons to stabilise, reconfigure and activate the feeding network in a polycyclic manner, the OC interneurons may also modulate the effectiveness of other modulatory feeding interneurons, like the SO, which drive fictive feeding for as long as they are depolarised. Stimulating the OC 4-10s before stimulating the SO significantly improves the response to SO depolarisation (Elliott and Vehovszky, 2000).
Here again, it is characteristic that the modulation is polycyclic, i.e. it lasts over several feeding cycles. This mechanism may contribute to the persistence of the feeding behaviour of intact snails after the feeding stimulus has terminated.
A possible cellular mechanism for the modulatory effect of the OC interneuron on other feeding connections is heterosynaptic facilitation. In particular, it may modulate the synaptic connections between the modulatory SO interneuron (which triggers feeding) and its followers, including the excitatory synapses with pattern-generating N1M protraction phase interneurons (Vehovszky and Elliott, 2001b). Thus preceding OC activity increases the ability of SO to trigger feeding pattern. When the feeding pattern is on (but does not reach its maximum rate), the longer- lasting modulatory effect on protrac- tion phase interneurons further increase the rate of the feeding rhythm.
Thus the modulation of the molluscan feeding network by octopaminergic neurons has many exciting aspects, and definitely supports the idea, that octopamine is not just for the arthropods. A wider significance for octopamine is suggested by the recent report from an annelid, the leech, for a significant role for octopamine (Mesce et al, 2001). The renewed interest in octopamine in vertebrates (Borowsky et al, 2001; Sherman and Chole, 2001; Rudling et al, 2000) implies that work on simple, but easily accessible molluscan systems will continue to be important.
Reference List
1 Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C (2001) Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98: 8966-8971.
2 Eisen JS, Marder E (1984) A mechanism for production of phase shifts in a pattern generator. J Neurophysiol 51: 1375-1393.
3 Elekes K, Voronezhskaya EE, Hiripi L, Eckert M, Rapus J (1996) Octopamine in the developing nervous system of the pond snail, Lymnaea stagnalis L. Acta Biol Hung 47: 73-87.
4 Elliott CJH, Vehovszky A (2000) Polycyclic neuromodulation of the feeding rhythm of the pond snail Lymnaea stagnalis by the intrinsic octopaminergic interneuron, OC. Brain Res 887: 63-69.
5 Evans CG, Alexeeva V, Rybak J, Karhunen T, Weiss KR, Cropper EC (1999) A pair of reciprocally inhibitory histaminergic sensory neurons are activated within the same phase of ingestive motor programs in Aplysia. J Neurosci 19: 845-858.
6 Hiripi L, Vehovszky A, Juhos S, Elekes K (1998) An octopaminergic system in the CNS of the snails, Lymnaea stagnalis and Helix pomatia. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 353: 1621-1629.
7 Mesce KA, Crisp KM, Gilchrist LS (2001) Mixtures of octopamine and serotonin have nonadditive effects on the CNS of the medicinal leech. J Neurophysiol 85: 2039-2046.
8 Roeder T (1999) Octopamine in invertebrates. Prog Neurobiol 59: 533-561.
9 Rudling JE, Richardson J, Evans PD (2000) A comparison of agonist-specific coupling of cloned human alpha (2)- adrenoceptor subtypes. Br J Pharmacol 131: 933-941.
10 Sherman BE, Chole RA (2001) Effects of catecholamines on calvarial bone resorption in vitro. Ann Otol Rhinol Laryngol 110: 682-689.
11 Vehovszky A, Elliott CJH (2000) The octopamine- containing buccal neurons are a new group of feeding interneurons in the pond snail Lymnaea stagnalis. Acta Biol Hung 51: 165-176.
12 Vehovszky A, Elliott CJH (2001a) Activation and reconfiguration of fictive feeding by the octopamine containing modulatory OC interneurons in the snail Lymnaea. J Neurophysiol 86: 792-808.
13 Vehovszky, A. and Elliott, CJH (2001b) Heterosynaptic facilitation in the feeding system of the snail Lymnaea by the octopaminergic OC interneurons. J Physiol (Bristol meeting abstract).
14 Vehovszky A, Elliott CJH, Voronezhskaya EE, Hiripi L, Elekes K (1998) Octopamine: A new feeding modulator in Lymnaea. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 353: 1631-1643.
Acknowledgements
We should like to thank BBSRC and The Wellcome Trust for their support.