
Physiology News Magazine
Short-term plasticity has a long synaptic history
Short- and long-term synaptic plasticity are dynamically linked, and this interplay helps to increase the information storage capacity of a synapse reports Yuji Ikegaya
Features
Short-term plasticity has a long synaptic history
Short- and long-term synaptic plasticity are dynamically linked, and this interplay helps to increase the information storage capacity of a synapse reports Yuji Ikegaya
Features
Yuji Ikegaya
Laboratory of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, University of Tokyo, Japan
https://doi.org/10.36866/pn.61.24
The rivers are running through the years, yet they are not the same water anymore.

This famous Japanese aphorism, written in the 13th century by the classical essayist Chomei Kamono, means that the rivers stay put in appearance but their material content (or inner state) is different. New data indicates that this principle holds true for synaptic transmission in the brain.

Synaptic efficacy is dynamic. For instance, when closely spaced action potentials reach a presynaptic terminal, the synapse does not transmit them identically to a postsynaptic neuron. This form of synaptic plasticity, termed short-term plasticity, is diverse (Fig. 1). At facilitating synapses, the postsynaptic responses to later spikes in repetitive presynaptic firing are larger than that to the first one, whereas at depressing synapses, they are smaller. Whether a synapse is facilitating or depressing depends upon the type of synapse. Hippocampal mossy fibre-CA3 synapses and climbing fiber-Purkinje cell synapses are typically facilitating, whereas parallel fiber-Purkinje cell synapses display depression. However, the biophysical mechanisms underlying short-term plasticity are multiple and complex, and therefore in many types of synapses, including hippocampal Schaffer collateral-CA1 synapses, these two forms of plasticity, i.e., facilitation and depression, often coexist, resulting in complicated profiles of short-term plasticity.
In addition to short-term plasticity, central synapses often show long-term plasticity, that is they are capable of increasing or decreasing their efficacy of transmission in response to brief repetitive synaptic activation and thereafter maintaining the changed efficacy for a long time. The temporal pattern of synaptic stimulation determines whether synaptic efficacy is strengthened (long-term potentiation, LTP) or weakened (long-term depression, LTD). Long-term plasticity represents long-lasting ‘memory’ at the sub-neuronal level and is widely believed to underlie learning and memory at the behavioural level.
Interestingly, the induction of long-term plasticity influences the profile of short-term plasticity. This interplay between two forms of synaptic plasticity is called redistribution of synaptic efficacy (RSE). The induction of LTP and LTD causes an increase and decrease in the depressing properties of synapses, respectively (Markram & Tsodyks, 1996; Sjöström et al. 2003). For an extreme example of LTP at neocortical synapses, short-term depression is augmented to a point at which the elevated synaptic efficacy disappears in later responses to highfrequency presynaptic firing (Markram & Tsodyks, 1996). Therefore, LTP and LTD do not simply amplify or attenuate synaptic transmission but rather transform (or filter) the content of information conveyed by spike discharges.
Strangely, however, RSE seems to be absent in hippocampal synapses (Pananceau et al. 1998; Selig et al. 1999; Buonomano, 1999). At these synapses, LTP is likely to equally increase all sequential responses to repetitive presynaptic stimulation, i.e., no change in the degree or direction of short-term plasticity. It has been unclear whether this apparent discrepancy is due to the difference in brain regions or stimulation protocols for LTP induction. Our new data now indicate that the latter is the case (Yasui et al. 2005).
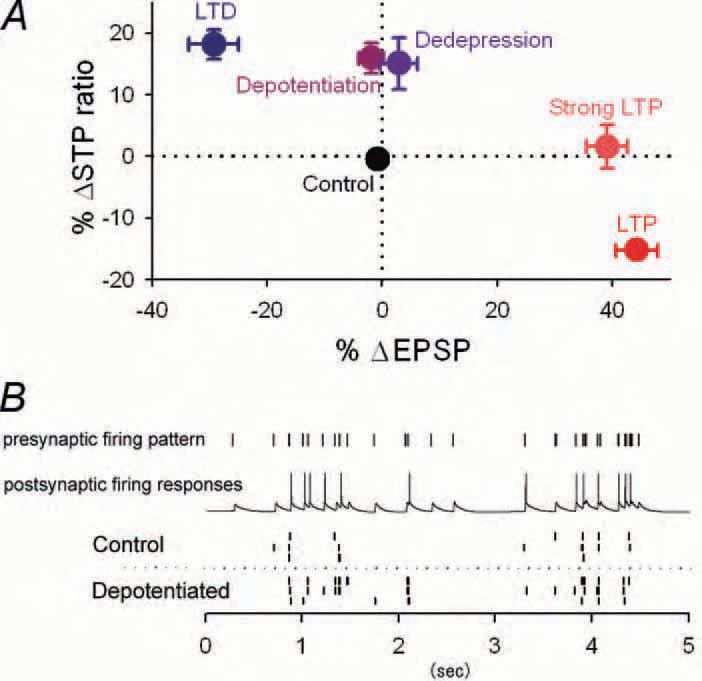
Using hippocampal slice preparations, we confirmed the previous findings that at Schaffer collateral-CA1 synapses, LTP was not accompanied by RSE. But this was true only if tetanic stimuli strong enough to induce saturated LTP were repeated four times. When the same tetanic stimulation was applied once, RSE was evident, that is, depressing synapses became more depressing after the LTP induction (Fig. 2A). Because LTP was already saturated by one tetanus under our experimental conditions, the synaptic efficacy for the initial spike in burst firing was no more changed by additional three tetani. This means that the states of these synapses that experienced one and four tetani are distinguishable only by RSE, i.e. the degree of short-term plasticity. Thus, synapses with apparently saturated LTP are still capable of encoding information by changing their profile of short-term plasticity. In this respect, we emphasize that RSE extends the information storage capacity of a synapse.
Similar phenomena take place after reversal of LTP (depotentiation) and LTD (dedepression) (Fig. 2A). Synapses potentiated by tetanic stimulation are believed to return to the basal conditions, i.e. the pre-tetanus state, by receiving subsequent lowfrequency stimulation. We found that such ‘depotentiated’ synapses were still accompanied by RSE although apparent synaptic strength, monitored by singlepulse stimulation, readily came back to baseline. Thus, depotentiation only ostensibly erases LTP, but the depotentiated synapses continue to convey information about the past plasticity, representing a unique functional state that differs from either naïve states or LTP, i.e., another level of plasticity. In fact, depotentiated synapses responded differently froma natural pattern of presynaptic activity, as compared with control synapses (Fig. 2B).
We thus conclude that short-term plasticity at central synapses is highly dynamic and could serve as a historic record of synaptic plasticity. This predicts a novel syntax of circuit operations, i.e. state-dependent propagations of neural signals. Elucidating RSE would reveal the computational significance of synaptic modifications.
References
Buonomano DV (1999). Distinct functional types of associative longterm potentiation in neocortical and hippocampal pyramidal neurons. J Neurosci 19, 6748-6754.
Markram H & Tsodyks M (1996). Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382, 807-810.
Pananceau M, Chen H & Gustafsson B (1998). Short-term facilitation evoked during brief afferent tetani is not altered by long-term potentiation in the guinea-pig hippocampal CA1 region. J Physiol (Lond) 508, 503-514.
Selig DK, Nicoll RA & Malenka RC (1999). Hippocampal long-term potentiation preserves the fidelity of postsynaptic responses to presynaptic bursts. J Neurosci 19, 1236-1246.
Sjöström PJ, Turrigiano GG & Nelson SB (2003). Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641-654.
Yasui T, Fujisawa S, Tsukamoto M, Matsuki N & Ikegaya Y (2005). Dynamic synapses as archives of synaptic history: State-dependent redistribution of synaptic efficacy in the rat hippocampal CA1. J Physiol (Lond) 566,143-160.
