Physiology News Magazine
The story of when kidneys fail:
The evolution of haemodialysis technology
Features
The story of when kidneys fail:
The evolution of haemodialysis technology
Features
Andrew Davenport, Royal Free Hospital and University College London, UK
https://doi.org/10.36866/pn.121.26
The products of cellular metabolism accumulate in patients with chronic kidney disease with potentially toxic effects, and today there are around 3 million patients with chronic kidney disease treated by dialysis worldwide. The term dialysis was first introduced by the Scottish chemist Thomas Graham, who reported in 1861 that crystalloids could be separated from colloids in solution by using a vegetable parchment acting as a semipermeable membrane. Almost 50 years later, Abel and colleagues in Baltimore employed a “vivi-diffusion” apparatus using a collodion membrane, later termed “artificial kidney” to perform the first dialysis treatment in dogs. Today, renal replacement therapy is a standard component of any modern nephrology and critical care unit, and as technology progresses, we get ever closer to developing wearable and implantable miniaturised artificial kidney devices.
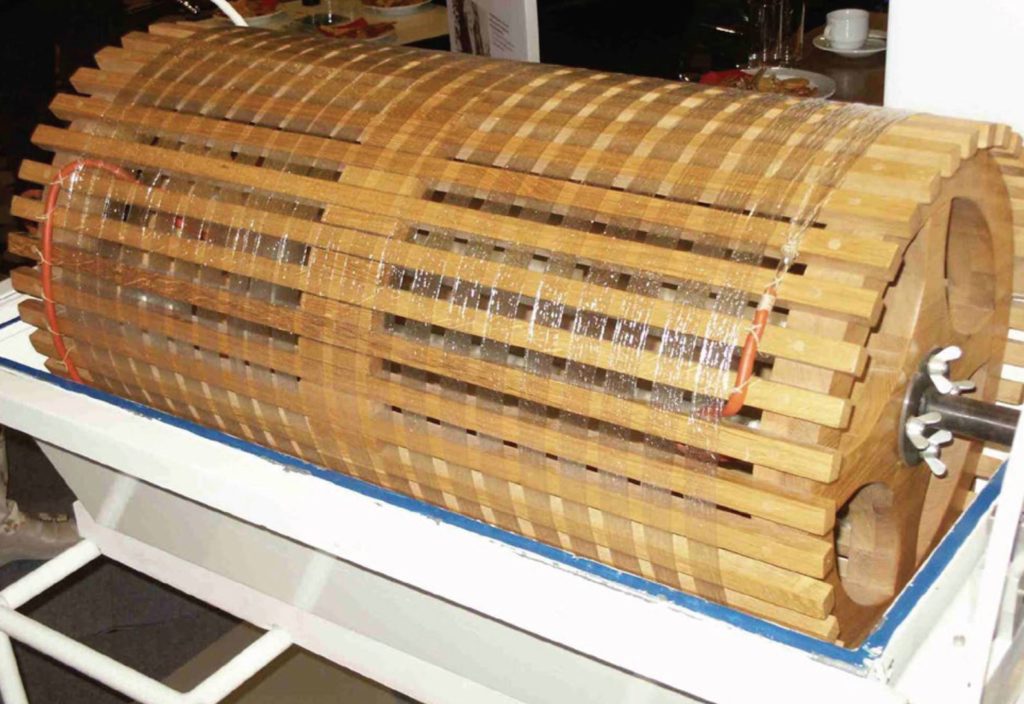
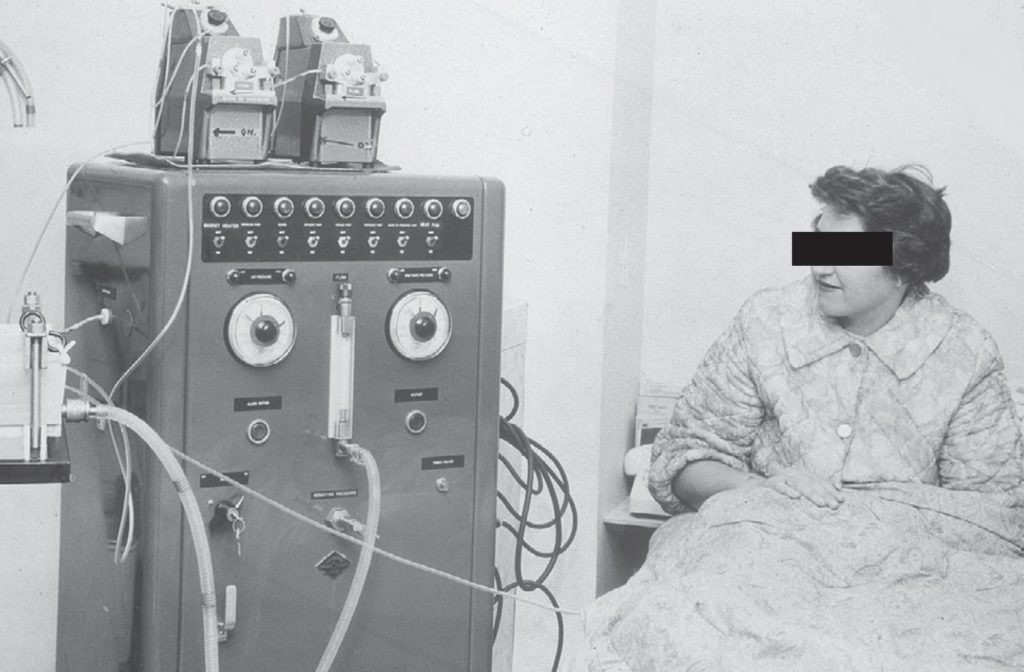
The first dialysis in a patient was performed by Haas in 1924 in Germany, using a collodion membrane and heparin anticoagulation, but he discontinued his trials in 1928 due to disappointing results. During the Second World War, acute kidney injury caused by rhabdomyolysis following crush injuries after bombings was recognised and this led to attempts to treat patients with both peritoneal dialysis and haemodialysis. Solute clearances with peritoneal dialysis were inefficient, and Willem Kolff in the Netherlands, who built a “rotating drum kidney” (Fig. 1) with a cellophane membrane, reported the first recovery of a patient with acute kidney injury treated by haemodialysis in 1945 (Kolff et al., 1997). Although Kolff’s dialyser could remove urea and other small solutes including potassium by diffusion, it could not provide ultrafiltration, and required the continued injection and then withdrawal of 50 mL blood aliquots. Neils Alwall in Lund, Sweden saw the potential for haemodialysis to become a life-sustaining treatment for patients with chronic kidney disease and led developments in both dialyser design and dialysis machine technology. However, chronic haemodialysis only became reality in 1960, when Scribner and colleagues designed an external arterio-venous by-pass made of Teflon® tubing, so allowing permanent access to the bloodstream (Fig. 2), and this was later improved in 1962 by the introduction of the arterio-venous fistula, first described by Cimino and Bresica (Agarwal et al., 2019).
Although haemodialysis is now an established treatment for patients with chronic kidney disease, performed in hospitals, stand-alone satellite centres or at home, the 5-year survival of haemodialysis patients is less than that for many patients with some of the more common solid organ malignancies, with most deaths due to cardiovascular disease. Globally, the majority of patients are treated three times a week, with each haemodialysis session lasting around 4 hours, which is not only an “unphysiological” schedule compared with normal renal function, but also introduces iatrogenic (i.e. caused by medical treatment) adverse effects generated by direct contact of blood with the dialyser surface material and blood tubing. Although electrolyte balance and control of acid–base balance can be achieved by regulating the concentrations in the dialysis water, during dialysis there are major fluxes of electrolytes with potassium and magnesium moving out from the patient’s blood, and bicarbonate and calcium moving from the dialysate into the patient’s blood. These fluxes in electrolytes can lead to changes in the electrocardiograph, particularly lengthening of the QT interval, which may predispose patients to cardiac arrhythmias, typically supraventricular ectopics and atrial fibrillation.
A consequence of the “unphysiological” schedule of the sessions is the accumulation of metabolic waste products from the end of one haemodialysis session to the start of the next treatment. Urea, which is derived from protein catabolism and recycling of amino acids has the highest concentration of any of the retained solutes. As urea is a small uncharged molecule it can diffuse rapidly, and so is very efficiently cleared during haemodialysis. This is achieved by maximising the relative plasma and dialysis water concentration gradient with relatively fast countercurrent flows using a large surface area dialyser comprising narrow diameter capillary fibres.. So, plasma urea clearance is much faster than urea movement from the cells into the plasma. In addition, as haemodialysis has a catabolic effect, due to the inflammatory response induced by the contact of blood with components of the extracorporeal circuit, intracellular urea generation is increased during treatment. Water therefore moves back into cells to counter this concentration gradient, with water moving through aquaporin channels some 20–30 times faster than urea leaves cells by urea transporters. Therefore, in clinical practice, post-haemodialysis most patients leave the dialysis unit with some cerebral white matter oedema and increased brain water (Waltersl et al., 2001), a condition known as dialysis equilibrium syndrome. Fortunately, most patients just feel tired, and take time to recover from the session, but occasionally patients may have a seizure or develop focal neurological signs.
Urea itself is not very toxic, as studies adding urea to the dialysis water to prevent the dialysis equilibrium syndrome exposed patients to very high blood urea concentrations without causing many of the symptoms associated with chronic kidney disease (Johnson et al., 1972). Urea can dissociate in plasma water to cyanate, which can react with proteins to form carbamylated haemoglobin or carbamylated albumin. However, unlike glycosylation in diabetics, which can result in a permanently glycated Amadori end-product, carbamylation is a reversible process, and so levels rapidly fall post-dialysis. As urea per se is not thought to be the “toxic” waste product of metabolism that leads to the signs and symptoms of end-stage kidney disease, this led to the search for other waste products of metabolism that cause toxicity. The EuroTox group have identified more than 100 potential compounds (Vanholder et al., 2008), and some of these potential toxins are of middle molecular weight, and not so readily removed by diffusion. For example, urea (64 Da) diffuses 18 times faster than β2 microglobulin (11,800 Da), so, one way of increasing larger solute clearances would be to increase the pore size of the dialyser membrane. However, simply increasing the pore size may then lead to increased losses of albumin, so there are technical issues to overcome to develop membranes that can allow diffusion of larger solutes but restrict albumin losses. An alternative is to add a convective movement of water, such that as blood is pumped through the dialyser a negative hydrostatic pressure is applied by the dialysis machine, and so water is pulled out from the plasma and any solute in the plasma water small enough to pass through the pores will now be removed with the water flux. So, with convection urea and β2 microglobulin are now removed equally, whereas with diffusion urea moves 18 times faster than β2 microglobulin. Combining diffusive and convective transport is termed haemodiafiltration, and studies (particularly those using higher volumes of convective clearance with haemodiafiltration) have shown greater patient survival, and in particular lower cardiovascular mortality for haemodiafiltration compared with standard haemodialysis (Nubé et al., 2017).
However, not all of the potential toxins currently identified are free in plasma water to be readily cleared by dialysis, and those bound to proteins have limited clearance by dialysis. Some of these putative toxins, including indoxyl sulphate (IS) and trimethylamine N-oxide (TMAO) have been shown to be associated with endothelial damage and cardiovascular disease. Granting that dialysers can be redesigned to include sorbents to remove these compounds, this adds costs and complexity to manufacturing processes. However, as these compounds are derived from amino acid, choline, betaine, and carnitine metabolism by bacteria in the large intestine, therefore alternative ways of reducing these toxins are being investigated, as patients with chronic kidney disease who consume a plant-based diet have been reported to have lower levels of these protein-bound toxins (Kandouz et al., 2016), and other potential toxins including deposition of advanced glycosylation end-products in the skin. Ultimately altering the gut microbiome by dietary interventions or medicinal compounds, or using absorbents to remove toxins, may be more practical ways of reducing these toxins.
In addition to the accumulation of waste products of metabolism, as patients may not pass any urine, patients gain weight from the end of one haemodialysis session to the start of the next session. Thus, a key objective of a dialysis treatment is to restore volume homeostasis and prevent left ventricular hypertrophy. Fluid weight gains are not limited to an expansion of the vascular compartment, as patients have a gain in extravascular lung water, and furthermore, magnetic resonance scanning has demonstrated an increase in both skeletal and cardiac muscle water content. Thus, during haemodialysis, fluid can only be removed from the plasma, but the majority of fluid gained between dialysis sessions is extravascular, so to maintain plasma volume the fluid has to move from extracellular and intracellular compartments in order to restore plasma volume. This compensatory refilling will depend on plasma tonicity (sodium and albumin concentration and haematocrit) and vascular permeability. However, for most patients there will be a contraction of the plasma volume following dialysis, as shown by a reduction in cardiac chamber sizes, as the rate of fluid removal from the plasma exceeds the refilling rate in the majority of patients (Kotecha et al., 2019). As such, patients may then develop hypotension if their cardiovascular response cannot compensate for any reduction in effective plasma volume. At the start of dialysis, passage of blood across the dialyser membrane and through the extracorporeal circuit generates an inflammatory reaction with the production of complement, leukocytes, macrophages, and bradykinin, causing an initial leukocyte and platelet sequestration in the pulmonary capillaries with a reduction in oxygen saturation, which then resolves. In addition, blood supply to the skin, liver, mesentery, muscle, and kidney all fall, with blood pooling in the larger-capacitance veins. This can lead to an increase in body core temperature, and if the body overheats, can potentially lead to vasodilatation and hypotension. Accordingly, some centres cool the dialysis water to increase heat exchange thus preventing a rise in core body temperature.
In the UK, the median age of the haemodialysis population is now aged in their mid-60s, with almost 50% of patients having a diagnosis of diabetes, therefore cardiovascular regulatory responses may be impaired and intra-dialytic hypotension remains the most common complication of out-patient treatments. These changes in blood supply can lead to a reduction in perfusion of both the heart and brain, which reverse after the dialysis session has ended. As for the heart, episodes of reversible cardiac stunning, whereby a section of the left ventricle temporarily stops contracting, are more likely to occur in patients with pre-existing cardiac disease. Similarly, for the brain, a reduction in cerebral blood supply and increased areas of white matter ischaemia are more likely to occur in the older patient, and it is suggested that repetitive episodes of cerebral ischaemia may account for the rapid decline in cognitive function reported in elderly patients initiating haemodialysis. However, this decline in cognitive function may not just be limited to the older patient, as cognitive function did not improve following renal transplantation in patients who had been treated by haemodialysis for more than 3 years. In addition, haemodialysis patients have a 10-fold increase risk of ischaemic stoke compared with the general population (Findlay et al., 2015). Whether this risk is due to the high prevalence of pre-existing hypertension, or due to the rapid changes in blood pressure during dialysis, remains to be determined.
If many of the adverse effects of haemodialysis are due to the intermittent nature of the treatment and rapid changes in solutes and reduced tissue blood supply, then daily treatments or continuous treatments could potentially improve patient outcomes. Indeed, more patients die after the longer weekend break between dialysis session. Studies using implantable cardiac monitors suggest that most deaths are associated with bradyarrhythmias, thought to be induced by the combination of hyperkalaemia and volume overload due to the greater time between dialysis sessions in the three times a week dialysis schedule. The Frequent Hemodialysis Network (FHN) study investigated the effect of dialysing 6 days a week compared with the conventional three-times per week paradigm (Tamura et al., 2013). Two trials were conducted: one comparing short daily dialysis and the other longer nocturnal sessions, both against the standard schedule. Although the short daily haemodialysis trial showed an improvement in left ventricular mass for those who initially had left ventricular hypertrophy, no benefits were observed on depression or nutrition. In particular, there were no benefits observed in terms of psychological performance, including attention, psychomotor speed, memory, or verbal fluency (Tamura et al., 2013).
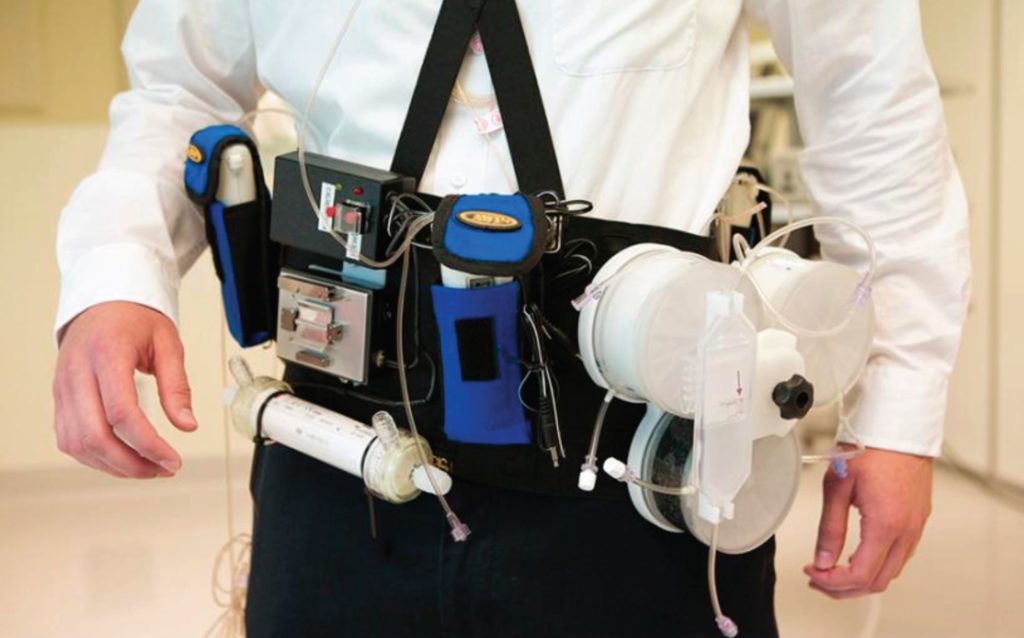
The alternative approach would be to develop a continuous form of haemodialysis (Davenport, 2015). Although a wearable device would not be suitable for all patients, an implantable device could potentially be offered to many. Investigators have considered designs based on the glomerulus and renal tubule structure. Studies to date have developed a highly permeable membrane design, similar to a storm drain in structure, producing a high-volume filtrate, but technical problems have yet to be overcome, in terms of developing a reliable implantable vascular access and then designing and constructing a filtration system to reabsorb the large volumes of fluid filtered by the nanomembrane. As such, greater progress has been made taking a conventional haemodialysis machine and then miniaturising the technology to develop a wearable device (Fig. 3). Initial safety trials have shown that such a device can be safe and, provided continuous clearance is maintained, it achieves middle molecule and protein-bound toxin clearance with patient recovery time being instantaneous (Davenport et al., 2007). These devices have been tested for up to 24 hours, and further trials have been planned to extend treatment times up to 7 days, including treatment at home (Gura et al., 2016).
References
Agarwal AK et al. (2019). Innovations in vascular access for hemodialysis. Kidney International 95(5), 1053–63. https://doi.org/10.1016/j. kint.2018.11.046.
Davenport A (2015). Portable and wearable dialysis devices for the treatment of patients with end-stage kidney failure: Wishful thinking or just over the horizon? Pediatric Nephrology 30(12), 2053–60. https://doi.org/10.1007/s00467-014-2968-3.
Davenport A et al. (2007). A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet 370(9604), 2005–10. https://doi. org/10.1016/S0140-6736(07)61864-9.
Findlay MD et al. (2015). Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke 46(9), 2477–81. https://doi. org/10.1161/STROKEAHA.115.009095.
Gura V et al. (2016). A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 1(8), e86397. https://doi.org/10.1172/jci.insight.86397.
Walters RJ et al. (2001). Haemodialysis and cerebral oedema. Nephron 87(2), 143. https://doi. org/10.1159/000045903.
Johnson WJ et al. (1972). Effects of urea loading in patients with far-advanced renal failure. Mayo Clinic Proceedings 47(1), 21–9.
Kandouz S et al. (2016). Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodialysis International 20(4), 610–7. https://doi. org/10.1111/hdi.12414.
Kolff WJ et al. (1997). The artificial kidney: a dialyser with a great area. 1944. Journal of the American Society of Nephrology 8(12), 1959–65. https://doi. org/10.1111/j.0954-6820.1944.tb03951.
Kotecha T et al. (2019). Acute changes in cardiac structural and tissue characterisation parameters following haemodialysis measured using cardiovascular magnetic resonance. Scientific Reports 9(1), 1388. https://doi.org/10.1038/s41598-018-37845-4.
Nubé MJ et al. (2017). HDF Pooling Project investigators. Mortality reduction by post-dilution online-haemodiafiltration: a cause-specific analysis. Nephrology Dialysis Transplantation 32(3), 548–55. https://doi.org/10.1093/ndt/gfw381.
Tamura MK et al. (2013). Frequent Hemodialysis Network (FHN) Trial Group. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American Journal of Kidney Diseases 61(2), 228–37. https://doi. org/10.1053/j.ajkd.2012.09.009.
Vanholder R et al. (2008). A bench to bedside view of uremic toxins. Journal of the American Society of Nephrology 19(5), 863–70. https://doi. org/10.1681/ASN.2007121377.