Physiology News Magazine
Time is of the essence:
Thrombolysis and thrombectomy treatment of acute ischaemic stroke
Features
Time is of the essence:
Thrombolysis and thrombectomy treatment of acute ischaemic stroke
Features
Rosanna Rossi, Andrew Douglas, Ciara Tierney & Karen Doyle, SFI Research Centre for Medical Devices (CURAM), National University of Ireland, Galway, Ireland
https://doi.org/10.36866/pn.121.19
Stroke, a leading cause of death and disability worldwide, is caused by deprivation of oxygen and nutrient supply to the brain, resulting in loss of brain cells within minutes, and causing neurological dysfunction. About 15% of strokes are haemorrhagic, but the majority (85%) are ischaemic attacks, caused by blockage of a cerebral vessel by a blood clot. Over 100,000 people in the UK and 10,000 people in Ireland suffer a stroke per year, with a mortality rate of approximately 40% (NICE, 2019; National Office of Clinical Audit, 2019).
Prior to the late 1990s, there was a lack of specialist acute stroke treatment available. Over the last 20 years, acute ischaemic stroke treatment has improved considerably, first through the approval of a thrombolytic drug to dissolve blood clots, and more recently through the development of mechanical thrombectomy as an intervention to remove the clot.
Thrombolysis
Blood clots form in response to vessel injury in a process that starts with formation of a loose platelet plug, followed by activation of the coagulation cascade resulting in formation of a fibrin mesh that strengthens the clot. Clot breakdown occurs through the activation of the zymogen plasminogen to form plasmin, a serine protease that is a powerful fibrinolytic. Plasmin cleaves fibrinogen and fibrin-rich diffuse networks in clots, thereby dissolving the clot (NINDS tPA Study Group, 1995). Cleavage of plasminogen to form plasmin occurs through the action of tissue plasminogen activator (tPA), which is slowly released by endothelial cells at the site of injury.

The first paper investigating the potential of a thrombolytic agent as a stroke treatment was published in 1958 (Sussman and Fitch, 1958). In this paper, Sussman and Fitch treated three patients with intravenous plasmin over a period of 6 days, leading to notable symptomatic improvement in one patient. However, due to increased risk of intracerebral haemorrhage, for many years the potential of thrombolytic agents to treat acute ischaemic stroke was not advanced to the clinic.
This changed in 1995, when a key clinical trial proved tPA to be beneficial (NINDS tPA Study Group, 1995). Subsequent approval y the US Food and Drug Administration (FDA) in 1996 led to the first major clinical development in the treatment of acute ischaemic stroke. Alteplase is a recombinant form of tPA (rtPA), identical to the tPA produced by endothelial cells in the human body. Alteplase has a very short life of 4-6 minutes, so it must be administered intravenously, and it is approved to treat stroke worldwide in an acute care setting. Alteplase minimises the likelihood of disability 3 months after treatment by 30%. Related thrombolytics, such as tenecteplase, which is a tPA variant that has a longer half-life, which can be administered as a bolus injection and also has greater fibrin specificity.
Tenecteplase is not yet approved for acute ischaemic stroke treatment worldwide although clinical trials are ongoing and promising.
Although tPA was, without doubt, a paradigm shift in acute ischaemic stroke treatment, it has some limitations and drawbacks, notably increased risk of haemorrhage. There is a short safe window for treatment in eligible patients (4.5 hours from onset of symptoms), resulting in only a small number of stroke cases being treated. In Ireland, current data shows that only 11% of acute ischaemic stroke cases receive thrombolysis (National Office of Clinical Audit, 2019).
Thrombectomy
The procedure of mechanical thrombectomy involves introducing a medical device into the vasculature via the groin, and threading through the heart to reach the cerebral vasculature. There are two main types of medical device used: aspiration catheters and retrievable stent devices (known as stentrievers) (Fig. 1). In 2015, five separate randomised, controlled clinical trials using early prototype devices for mechanical thrombectomy showed positive results in acute ischaemic stroke patients with large vessel occlusions (Palaniswami and Yan, 2015). In 2019, 9% of people who had an acute ischaemic stroke in Ireland received thrombectomy treatment (National Office of Clinical Audit, 2019), an increase from 7% in 2018. In the UK, in 2017, only 1% of acute ischaemic stroke cases had thrombectomy, with the intent to increase to 10% as soon as possible (NICE, 2019).
Aspiration
In aspiration thrombectomy, a catheter of appropriate internal diameter is advanced to the occluded vessel and the distal end of the catheter is placed proximal to the clot (Fig. 1B). A negative pressure is applied via syringe or pump and the clot is suctioned into the catheter. Historically, the use of direct, also known as contact, aspiration in acute ischaemic stroke treatment was limited by the lack of catheters large enough to provide sufficient aspiration, yet flexible and atraumatic enough to navigate the tortuous intracranial vasculature. With newer aspiration catheters, these obstacles have been overcome, prompting the inclusion of aspiration alone as a valid alternative treatment for stroke.
Stentrievers
The first generation of retrievable stent devices were minimally invasive, catheter-based devices designed to physically entrap the clot to facilitate its removal. Although many developments have occurred since the first stentriever prototypes, the general way they work remains the same. First, a microcatheter crosses the clot, and then, as the retrievable stent is unsheathed from the microcatheter, it deploys, integrating into the clot. After a short time (typically 2-4 min) of allowing the stent to interact with the clot, the stent and microcatheter are retrieved, pulling out the clot (Fig. 1A). Although still very new, developments in stentriever design have improved thrombus–stent interaction, leading to better recanalisation rates and better patient outcomes.
Stentrievers and aspiration catheters can be used individually or in combination. The combined approach has recently shown success, although the cost involved of using multiple devices may be prohibitive.
Future therapeutic strategies for acute ischaemic stroke pathology
The future of acute ischaemic stroke treatment depends on the success of two research-led approaches. The first approach is to improve our understanding of the molecular mechanisms of brain injury caused by stroke, ultimately leading to neuroprotective drugs. This is the holy grail, but it is not likely to be realised imminently.
A second approach, to improve understanding of the cerebrovascular aspects of stroke, could lead to the discovery of new therapeutic targets and stroke prevention strategies in a shorter timeframe. Furthermore, research to improve the therapeutic strategies that are already available, such as further improvements in thrombolytics as well as novel and more effective thrombectomy medical devices will improve acute stroke treatment within a very short timeframe, saving many lives, and improving the quality of life for many others and their families.
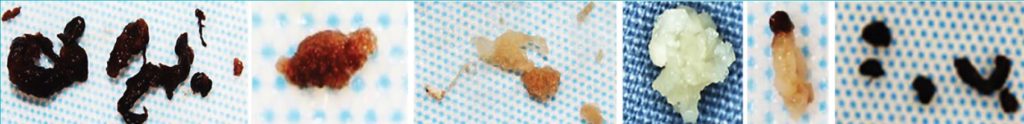
Retrieval of the clots that cause strokes has afforded researchers the opportunity to study their composition using histology and immunohistochemistry. Not all clots that cause strokes are the same. The main components in a blood clot are red blood cells, fibrin, platelets and white blood cells. However, the proportions of these main components can vary considerably (Fig. 2). Clots range from being red blood cell rich, with loose fibrin stands providing the scaffold to hold the structure together, to containing almost no red blood cells, instead densely packed with fibrin strands and platelets. Some clots contain other components such as collagen, and others are calcified.

The composition of the clot significantly influences the outcome for patients treated with both rtPA and mechanical thrombectomy devices. For example, there is evidence that clots that are red blood cell rich with loose fibrin strands respond best to tPA, which effectively reduces the size of those clots, while other clots are tPA resistant, and need to be removed by mechanical thrombectomy (Rossi et al., 2020). Other recent work in our lab has found that clot composition varies with stroke aetiology (Fig. 3) (Fitzgerald et al., 2020). There are still many unanswered questions, and much left to learn. Further interrogation of the clots that cause strokes will help to advance understanding of the causes of stroke, the pathophysiology of the cerebrovasculature in stroke and advance medical device design for even the most difficult to remove clots, improving acute stroke care.
References
Fitzgerald S et al. (2020). Per pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted-clot-area and histological composition. Journal of Neurointerventional Surgery. https://doi. org/10.1136/neurintsurg-2020-016966.
National Office of Clinical Audit (2019) Irish National Audit of Stroke – 2019 [Online]. National Office of Clinical Audit. Available at: https://www.noca.ie/ documents/irish-national-audit-of-stroke-2019.
NICE (2019). NICEimpact stroke [Online]. NICE. Available at: https://www.nice.org.uk/Media/ Default/About/what-we-do/Into-practice/ measuring-uptake/NICE-Impact-stroke.pdf.
NINDS tPA Study Group (1995). Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine 333, 1581–1587. https://doi. org/10.1056/NEJM199512143332401.
Palaniswami M, Yan B (2015). Mechanical thrombectomy is now the gold standard for acute ischemic stroke: Implications for routine clinical practice. Interventional Neurology 4(1-2), 18–29. https://doi.org/10.1159/000438774.
Rossi R et al. (2020). The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. Journal of Thrombosis and Thrombolysis 51(20). https://doi. org/10.1007/s11239-020-02279-1.
Sussman BJ, Fitch TS (1958). Thrombolysis with fibrinolysin in cerebral arterial occlusion. Journal of the American Medical Association 167, 1705-1709. https://doi.org/10.1001/ jama.1958.02990310011002.