Physiology News Magazine
Vacation Studentships
In 2013, The Society’s Vacation Studentship scheme funded 39 students to gain hands-on research experience in the lab of a Society Member. We asked each of these students to prepare a report summarising their experience, achievements and results. The reports were then reviewed by Members of the Education and Outreach Committee and two were selected for publication in Physiology News
Membership
Vacation Studentships
In 2013, The Society’s Vacation Studentship scheme funded 39 students to gain hands-on research experience in the lab of a Society Member. We asked each of these students to prepare a report summarising their experience, achievements and results. The reports were then reviewed by Members of the Education and Outreach Committee and two were selected for publication in Physiology News
Membership
Sam Bose
University of Bristol, UK
Jonathan Raby
University of Cambridge, UK
https://doi.org/10.36866/pn.93.50

Having developed an interest in cardiovascular physiology and electrophysiology during my first two years as an undergraduate, I was keen to gain laboratory experience in these fields of research. After approaching Andrew James (Andy) from the School of Physiology & Pharmacology at the University of Bristol and deciding upon a project, I applied to The Society for a Vacation Studentship to work in Andy’s lab over the summer. These studentships are designed to enable undergraduate students to undertake a research project lasting up to eight weeks during the summer vacation and cover living expenses for that period.
Our project aimed to investigate the potential for the anti-anginal agent, ranolazine, to be protective against atrial fibrillation. Atrial fibrillation is the most common arrhythmia and a major risk factor for stroke. Previous studies had highlighted an atrial-specific action of ranolazine, a property that would be beneficial due to the reduced risk of ventricular side effects. Ranolazine is a sodium channel blocker that inhibits a sustained component of the sodium current.
Our aim was to investigate how ranolazine affects atrial electrophysiology and the inducibility of atrial tachyarrhythmia (AT) during metabolic stress in Langendorff-perfused rat hearts.
The first couple of weeks of the project consisted mainly of training, both in the use of the laboratory equipment and the interpretation of electrophysiological recordings. Although the University of Bristol provides a high degree of practical training to undergraduates, the work I was doing for this project went far beyond anything I had experienced before. Hearts were excised from adult male rats, mounted on a modified Langendorff-perfusion apparatus and electrograms recorded from the surface of the left atrium using a 5×5 electrode array. Being a big fan of all things bespoke, I enjoyed the fact that this array consisted of platinum/iridium wires embedded in dental resin using an eppendorf tube as a mould! As a self-confessed geek, I don’t mind mentioning that the DIY nature of such equipment makes electrophysiology particularly appealing to me!
Having come to terms with the daunting setup of amplifiers, tubes and wires that I would be working with for the next eight weeks, the next task was to learn how to interpret the recordings. It took me many long hours of fiddling with wires and earth connections, and stimulating the heart at various current amplitudes before I was happy that I was able to distinguish actual electrograms from noise, baseline wander and stimulus artefact. However, once I knew what I was looking at and had confidence in my ability to interpret the traces, the experiments started to run smoothly and I was able to gather useful data.
The recording of electrograms enabled the measurement of both atrial effective refractory period and conduction velocity. The product of refractory period and conduction velocity provides the wavelength of excitation, increases in which are thought to provide protection against re-entrant arrhythmias. Our hypothesis was that although ranolazine would most likely cause a decrease in conduction velocity due to sodium channel block, the drug may also increase refractory period sufficiently to result in an overall increase in wavelength. In addition, we examined the effect of ranolazine on the inducibility of tachyarrhythmia during beta-adrenergically induced metabolic stress. Bursts of very rapid (>100 Hz) pacing were used to induce paroxysms of AT. Metabolic stress was produced by perfusion with the beta-adrenoreceptor agonist, isoprenaline.
Firstly, perfusion with isoprenaline markedly increased the inducibility of AT through reduction in the refractory period and wavelength. Ranolazine suppressed the development of AT during metabolic stress. However, this anti-arrhythmic effect was not correlated with changes in the wavelength of excitation. Although ranolazine prolonged the atrial effective refractory period and slowed conduction velocity, the drug did not completely prevent the metabolic stress-induced changes in these parameters. The study has opened the door to further research into the mechanism of suppression that was observed.
Being able to undertake this research project has confirmed my desire to pursue a career in research and has allowed me to experience firsthand what laboratory work involves. When the placement first started, I must confess to feeling a little out of my depth. I was scared of making silly mistakes or getting in the way of others. However I soon found myself gaining confidence and by the end of the project I felt at home in the lab. The work was challenging, but hugely satisfying when the results started to come through. My desire to pursue a PhD after graduating next summer is confirmed.
I would like to acknowledge the help and support of Andy James, Richard Bond, Jules Hancox and everyone from the University of Bristol Cardiovascular Research Laboratory with this project, as well as The Physiological Society for providing the funding that made it possible.

Over the summer of 2013 I spent eight weeks working on the molecular basis of acid pain sensation with Ewan Smith in the Department of Pharmacology, University of Cambridge. I am currently in my third year of an undergraduate medicine degree and wanted to gain some experience of the type of work and lifestyle associated with entering a research environment in order to decide whether pursuing a PhD and a career in research is right for me.
Before undertaking the project, my only previous experience of laboratory work was through practical sessions associated with my undergraduate degree, which have tended to be time-pressured and prescriptive. The studentship has given me a more realistic impression of what practical science is like, and I believe I have developed a better understanding of the patience and pragmatism required when undertaking research. In contrast to undergraduate practicals designed to be robust and predictable in outcome, sometimes the experiments and procedures I performed simply did not work (occasionally for no clear reason), and I was given some insight into the continuous process of troubleshooting and refinement that seems important for improving the chances of later success and the quality of data generated. I also experienced for the first time some of the excitement that stems from a novel, unexpected result and the challenge associated with formulating ideas and hypotheses that might explain the phenomena observed (by contrast, in undergraduate study the aim of experiments was generally to generate results consistent with established theory, and ‘deviant’ data were usually disregarded rather than explored).
My pre-conception of working in a lab held that it was a rather lonely existence, and that engaging in independent research could lead to a degree of isolation. However, my experience over the course of the studentship has shown me that a career in science can have far more of a ‘team feel’ than I had been expecting, and that there is great capacity for (and benefit in) collaborations within the lab, University or even internationally. The other members of the lab and department were extremely welcoming and generous with their time, and I enjoyed having the chance to get to know a particular department in the University as a research environment, and to interact with its members in a more personal, less one-directional way than had been possible during my undergraduate course.
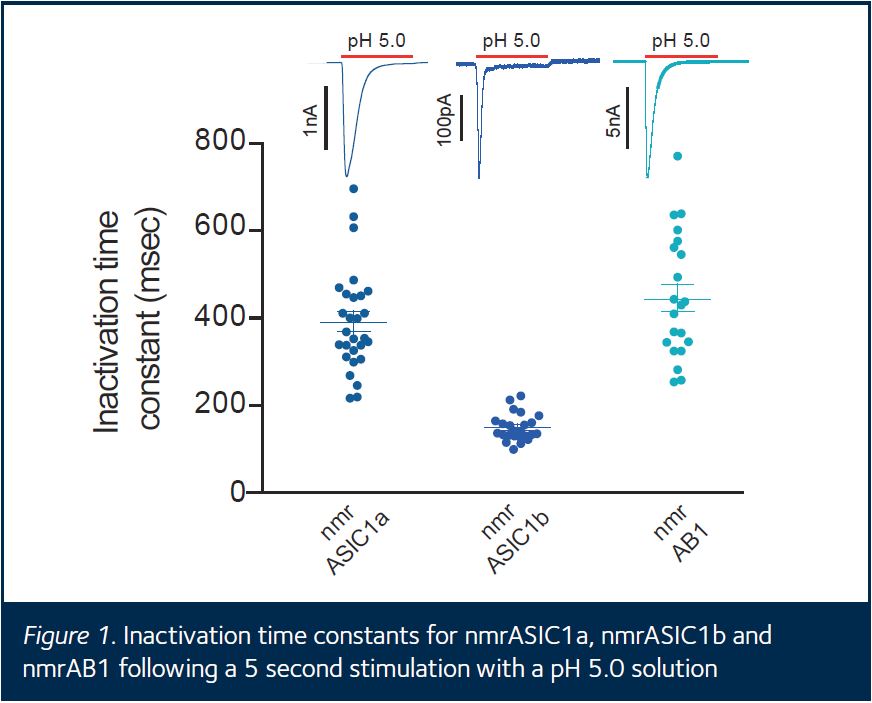
The aim of my project was to explore the structural bases of the functional differences between the two acid sensing ion channels (ASICs) produced by alternative splicing of the ASIC1 gene (ASIC1a and ASIC1b). Responses of ASIC1a and ASIC1b to low pH stimulation differ both quantitatively and qualitatively, and the splice variants differ in their first 172αα, which suggests that domains within this region (including the intracellular N-terminus of the protein) confer the different properties observed. To improve understanding of the link between structure and function of ASIC1 splice variants, I created a novel chimera of naked mole rat ASIC1a and ASIC1b in which part of the N-terminus region of nmrASIC1b was replaced with equivalent amino acids from nmrASIC1a (this was achieved by a polymerase chain reaction using primers that amplified only the regions of each splice variant that were to form the chimera). I was trained in a range of microbiological techniques that allowed me to transfect Chinese hamster ovary cells with plasmid vectors carrying either nmrASIC1b or the ‘AB1’ chimera. I then investigated the biophysical properties of each channel type using the whole-cell patch-clamp technique. The responses of the transfected cells to different low pH solutions were measured using various experimental protocols designed to characterise channel properties including response magnitude, inactivation time constant, current-voltage and dose-response relationships. In summary, the ‘AB1’ chimera behaved much more similarly to nmrASIC1a than to nmrASIC1b, suggesting that the N-terminus plays a critical role in channel function (Fig. 1 shows that the AB1 inactivation time constant is significantly different to that of nmrASIC1b and highly similar to that of nmrASIC1a).
The Physiological Society Vacation Studentship allowed me to undertake a research project that has been influential in shaping my career plans and scientific interests. Given the stimulation and satisfaction that I found in even a short period of time spent in the lab, I am now certain that I would find a career in science exciting and fulfilling. I am currently exploring the various routes by which I could pursue a PhD while continuing to progress in my medical career. The project I worked on has confirmed an interest in the physiology of pain sensation, which has meshed with a pre-existing clinical interest in anaesthesia. However, undertaking the project has also given me a better appreciation of the huge range of exciting research opportunities that exist within the physiological sciences, and so I intend to keep an open mind as to the exact nature of any PhD I may undertake for as long as possible.
I acknowledge the supervision of Laura-Nadine Schuhmacher and Ewan Smith.