
Physiology News Magazine
Working out aerobic fitness
Features
Working out aerobic fitness
Features
Ole Johan Kemi (1), Per Magnus Haram (2), Ulrik Wisløff (2,3), Øyvind Ellingsen (2,3)
1: Institute of Biomedical and Life Sciences, University of Glasgow, UK
2: Department of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway
3: St Olavs Hospital, Trondheim, Norway
https://doi.org/10.36866/pn.634.18

In the late 1990s, we combined cellular cardiology and exercise physiology to study the biological causes of why physical activity and exercise training are beneficial in both health and disease. Physical activity and exercise training increases function and prevents disease in the cardiovascular system, improves the outcome in patients with manifest disease and improves the prospects of rehabilitation. In fact, several sets of data strongly suggest that the level of aerobic fitness, as determined by metabolic equivalents or maximal oxygen uptake (VO2max), directly links to health and survival prospects, even when traditional risk factors are present or the patient is on conventional medical treatment (Myers et al. 2002, O’Neill et al. 2005). It is clear that changes originating from the cell or molecule of the heart and artery must accompany and possibly explain the beneficial effects.
Thus, we established experimental models to study research questions pertaining to biological mechanisms of fitness and health (Kemi et al. 2002, Wisloff et al. 2001). Today, the guidelines regarding exercise training as a therapeutic approach need refinement and fine-tuning. An important aspect is therefore that exercise and the effects on exercise capacity must be controlled, in terms of measuring VO2max accurately and in terms of how much exercise is performed causing the outcome. An intensity-controlled treadmill model is currently the only suitable model allowing this. Custom-made treadmills with metabolic chambers for both mice and rats were found to be reproducible for measuring oxygen uptake (VO2) over a range of running intensities. Also, ramp test protocols for measuring VO2max were established; highest values obtained at or around 25º inclination indicated that inclined running is necessary to provoke VO2max. Moreover, a good agreement between heart rate and VO2 up to maximal levels during running to exhaustion; but with maximal heart rate occurring after VO2max, and several-fold elevated blood lactate levels (peaking at 6-7 mM) and respiratory exchange ratios above 1.05 when reaching VO2max, strongly suggest that the animals were running to exhaustion, and that VO2max was assessed validly. Thus, the current procedures for assessing VO2 and VO2max by large resemble common protocols used by applied exercise physiology laboratories and clinical services working up athletes as well as patients. For instance, respiratory exchange ratios and blood lactate levels as described above suggest that the individual, whether it be man or mouse, is exercising above the aerobic window and well into where anaerobic metabolic processes kick in. These are therefore common criteria used to determine whether or not a ‘true’ VO2max is reached.
Next, we investigated the effects of prolonged high-intensity (85-90% of VO2max, 5 days/week) interval exercise training on the heart and artery, or the cells that make up those organs (Kemi et al. 2002, 2004, 2005, Wisloff et al. 2001). As VO2max was measured in each animal every week, the running speed was continuously adjusted to maintain the relative training intensity individually. 8-10 weeks of regular training improves VO2max by ~50-70%; slightly more in rats than mice. Correspondingly, maximal aerobic velocity, i.e. the running velocity where VO2max was reached, improved similarly. Moreover, the running economy, measured as the cost of oxygen to run at a certain speed, was reduced by ~20% during submaximal running velocities, whereas the respiratory exchange ratio was reduced by ~5% at those velocities. As for the heart, right and left ventricular masses increased 20-30%, which was supported by echocardiography recordings, whereas left ventricular cardiomyocytes increased in size by ~20%, due to proportional cell length and width increase. Cell contractile function, measured at physiological electrical stimulation frequencies, also improves after exercise training; the magnitude of shortening increased 3040%. In rats, this appears to be largely explained by improved Ca2+ sensitivity, rather than by more Ca2+ being available to the myofilament machinery, as is the case in mice (unpublished data). Detailed studies of the myofilament responsiveness to given free [Ca2+] in permeabilized cells support our data that less Ca2+ is needed to evoke the same response after training in rats (Diffee et al. 2001). Myocytes from the hearts of trained animals also contract and relax more quickly than those from untrained animals, which coincides with a more rapid increase and decay of the [Ca2+] transient. Taken together, the structural and functional adaptations of the cardiac muscle cell to long-term exercise explains at least partly why whole-heart function in trained athletes is better than in untrained; increased stroke volume and cardiac output being hallmarks of vigorous exercise over months and years. The cellular findings also provide possible reasoning for better diastolic filling of the ventricles and the concept of athlete’s heart.
With increased cardiac output, the artery needs to accordingly secure sufficient conductance of blood to the skeletal muscles. One way of doing so would be increasing the arterial structural diameter permanently, but an alternative way is to improve the vasodilatory capacity. The main vasorelaxive messenger is nitric oxide (NO), which when released from the endothelial cell ultimately reduces intracellular [Ca2+] in the smooth myocyte, causing relaxation. This pathway is stimulated by acetylcholine that speeds up NO production in the endothelium. We find that regular exercise training improves endothelial function, as the acetylcholine concentration evokes half-relaxation decreased ~4-fold in trained animals. There was no difference with an exogenous NO donor (nitroprusside) or after blocking NO synthase (L-NAME), adding further weight to the argument.
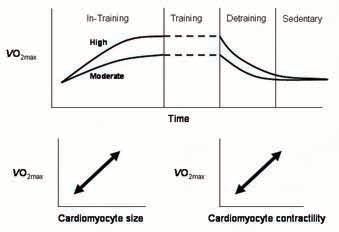
Given that regular exercise training considerably affects the morphology and physiology of the cell, we moved on to study the plasticity of the changes, by intervening with different exercise training programs. We compared the effects of high (85-90% of VO2max) vs. moderate (65-70%) intensity exercise, where 1 hour was allocated to exercise per day, 5 days/week (Kemi et al. 2005). Moderate intensity increased VO2max only ~half as much as high intensity, paralleled by similar reductions in the magnitude of changes on cardiac myocyte size, fractional shortening, Ca2+ sensitivity, and time-courses of the contraction-relaxation cycle and the [Ca2+] transient. Thus, the exerciseinduced adaptations in the cardiac myocyte depend upon intensity of exercise. Artery endothelial function had a slightly different dose-response relationship. Acetylcholine-mediated NO-dependent vasoreactivity increased with exercise, and reached nearmaximum level of adaptation with moderate exercise, and only a trend for higher sensitivity was observed between moderate and high intensity.
In a different study (Kemi et al. 2004), we investigated the time-course of adaptations during training and detraining, that is, how quickly do the changes occur when a high-intensity exercise program is implemented, and how quickly do the effects vanish after withdrawing regular exercise? Whereas it takes about 8-10 weeks of training for VO2max, cardiac myocyte size, contractile function and Ca2+ handling to adapt, most of these effects vanish very quickly when regular exercise is stopped. After regular exercise training for 10 weeks, half the increase in VO2max was lost within 2 weeks, most of the effects on cardiomyocyte structure and function disappeared within 2-4 weeks, and arterial improvement was completely lost within 2 weeks of detraining. Thus, exercise-induced beneficial adaptations appear vulnerable; what takes months to improve, only takes weeks to lose again if not properly maintained.
The rigorous control of exercise intensity and volume, as well as adaptations, in terms of VO2max is unprecedented in experimental studies; with this scheme, exercise training moves from ‘physical activity’ to an entity in which responses may be correlated to dose, or the cellular and molecular adaptations may be correlated to changes in VO2max or overall, whole-body function. We conclude that aerobic fitness is closely related to cardiomyocyte contractile capacity and acetylcholine-mediated endothelial function, and that the magnitude of training-induced adaptations depends on exercise intensity.
References
Diffee GM, Seversen EA, Titus MM (2001). Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. J Appl Physiol 91, 309-315.
Kemi OJ, Haram PM, Loennechen JP, Osnes, J-B, Skomedal T, Wisloff U, Ellingsen O (2005). Moderate vs. high intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res 67, 161-72.
Kemi, OJ, Haram PM, Wisloff U, Ellingsen O (2004). Aerobic fitness is associated with cardiomyocyte contractile capacity endothelial function in exercise training and detraining. Circulation 109, 28972904.
Kemi OJ, Loennechen JP, Wisloff U, Ellingsen O (2002). Intensity controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93, 1301-1309.
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE (2002). Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346, 793-801.
O’Neill JO, Young JB, Pothier CE, Lauer MS (2005). Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 111, 2313-2318.
Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO2max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280, H1301-H1310.
