Physiology News Magazine
Artificial blood transfusion:
A new chapter in an old story
Features
Artificial blood transfusion:
A new chapter in an old story
Features
Kohsuke Hagisawa, National Defense Medical College, Saitama, Japan
Manabu Kinoshita, National Defense Medical College, Saitama, Japan
Hiromi Sakai, Nara Medical University, Japan
Shinji Takeoka, Waseda University, Japan
https://doi.org/10.36866/pn.121.22
Among bodily substances, blood is particularly prominent. Conspicuous by its crimson colour, it not only permeates our bodies but also our societies and cultures, from religious customs to vampire literature to crime dramas. Our fascination with blood is unsurprising given that it plays crucial roles in our bodies (e.g. in transportation, homeostasis, and defence). And yet, despite around 118.5 million blood donations globally, the supply of this most ubiquitous of substances is often outstripped by demand. One solution to this shortage is the mass manufacturing of artificial substitutes, but many of these have failed to meet the safety standards and physiological needs, until recently.
As every physiology undergraduate learns, the centrifugation of blood reveals that it consists of three components: red blood cells (RBCs); a buffy coat that contains white blood cells (WBCs) and platelets; and plasma. RBCs contain haemoglobin (Hb) and work as a gas carrier, transporting oxygen and carbon dioxide between the lungs and tissues, as well as a pH buffer. WBCs recognise and remove foreign invaders, while platelets promote blood clotting. Together they maintain haemostasis and orchestrate our immune defences. Plasma, though mostly water, also contains proteins (e.g. albumin, globulin, and fibrinogen) and other solutes (e.g. gases, ions, and hormones), one of the most important of which is albumin, which maintains colloid osmotic pressure and blood volume essential for haemodynamic stability.

Given these vital functions, the anticipated effects of blood loss (haemorrhage) on our bodies are adverse. Indeed, depending on the degree of bleeding, haemorrhaging patients experience what clinicians refer to as “shock”, namely a drop in blood pressure (hypotension), a rise in heart rate (tachycardia), hypovolaemic-induced hypoperfusion of tissues, and a subsequent a lack of cellular oxygen delivery (ischaemia). In such cases, rapid fluid infusion and blood transfusion are required to replenish the lost components and restore haemodynamic stability and oxygen supply. Whilst transfusions are simple (blood from one person is transferred to another’s circulation intravenously), they remain indispensable for treating patients suffering from severe bleeds, anaemia, and cancer. In fact, it can be said that blood transfusions are a kind of cell therapy or tissue transplant (it is technically a connective tissue after all). It’s useful, therefore, to consider the history of blood transfusions, and how advancements in this therapy can save lives.
The history of blood donation
The transfusion story starts in the 1800s with the British obstetrician Dr James Blundell. Practising in London, Dr Blundell first implemented transfusion (using fresh, whole human blood) for the treatment of post-partum haemorrhage (i.e. excessive bleeding after childbirth; Blundell, 1829). He later demonstrated that haemophilia (a rare genetic disease that affects blood clotting) could be managed in a similar way. Despite these advances, transfusions persisted at the periphery of medicine for some time, mostly owing to a high risk of death. It wasn’t until the discovery of distinct human blood types in 1901 that the lethal complications of mismatched blood typing were resolved.
During the 20th century, great efforts were made to adapt the extraction and processing of blood, improving its long-term storage capacity and increasing its clinical adoption. For example, anticoagulants made it possible to safely store donated blood in a stable condition. Centrifugation enabled the fractionation of blood into its constituent parts. Once RBCs, WBCs, platelets, and plasma were able to be sorted and stored, they could be used independently, giving rise to component transfusion.
Now, the technology has developed further to fractionate albumin, globulins, and fibrinogen from plasma. Nevertheless, the contamination of donated blood by pathogens (e.g. hepatitis and HIV) remains an issue. Today, medical interviews of donors and advanced blood screening help ensure that most transfusion-transmitted infections are prevented. However, new emerging pathogens (e.g. SARS-CoV-2) threaten the present blood donation and transfusion system.
Honing blood transfusion techniques
Fluid treatment (including component transfusion) has saved many patients from haemorrhagic shock. And yet it can also increase dilutional coagulopathy (i.e. an impairment of blood clotting following the dilution of platelets in large volumes of fluid). To mitigate this, the concept of balanced transfusion has been introduced in the US whereby RBCs, platelets, and plasma are administered at a unit ratio of 1:1:1 in the early stages of transfusion (including prehospital transfusion; Holcomb et al., 2015). However, such fluid delivery of three components is not easy to prepare promptly, owing to the different storage conditions of each (e.g. packed RBCs are readily available from a refrigerator, but fresh frozen plasma takes at least 30 minutes to thaw before use). Likewise, logistical limitations exist because of the different shelf-lives of RBCs (3–6 weeks) and platelets (3–4 days); plus, platelet concentrates require shaking at 20–24˚C for preservation. This complex situation makes it hard for clinical staff to administer urgent transfusions simultaneously. Moreover, component transfusions require specific preservative solutions for each component, and severe trauma patients need to receive additional fluids (e.g. antibiotics, inotropes, nutrients). This non-negligible volume of fluids can cause a further rise in volume, leading to worsened dilutional coagulopathy and patient outcomes.
The use of blood transfusions are further complicated by the various inflammatory responses and organ damage they may evoke in the hosts. For example, Transfusion-Related Acute Lung Injury (TRALI) and Graft-versus- Host Disease (GvHD). To avoid such immune reactions, several modalities have been adopted, such as the irradiation of blood products.
Additionally, a recent paper demonstrated the enzymatic conversion of type A to type O blood (Rahfeld and Withers, 2020), which has the potential to boost the amount of the universal blood donor type available and minimise the risk of adverse reactions in those who receive it. As climate change continues, natural disasters will occur more frequently, and the care of mass casualties will become an inevitable consequence. Not only is it difficult to save a lot of injured victims of such events, but should the supply system of medical resources (including blood products) be broken, a larger number of fatalities will occur.
The future of blood transfusion: artificial blood
Hb has dual roles: oxyHb literally transports oxygen in the arteries and deoxyHb captures protons in the veins (a role important for pH buffering). The Bohr and Haldane effects are essential to these unique properties of Hb, whereby an increase of carbon dioxide in the blood promotes the displacement of oxygen from the Hb, and conversely the binding of oxygen to Hb during high oxygen concentration tends to displace carbon dioxide from the blood (Hall, 2015). Additionally, RBCs utilise the carbonic anhydrase to metabolise carbon dioxide into protons and bicarbonate for transportation. (Fig. 1).
Considering the current situation, additional innovation is required to improve blood transfusion as a therapy and make it more accessible to patients. Our laboratory is interested in the use of acellular Hb as a form of “artificial blood” to do just that given its unique properties.
Purified Hb was first tested as a substitute for RBCs in transfusions in the 1930s. However, “naked” Hb caused renal toxicity and adverse cardiovascular effects (presumably resulting from scavenging of nitric oxide; NO). Since the 1970s, cross-linked Hb, recombinant Hb, and polymer-conjugated Hb have been developed. But, most failed to progress to clinical trials because the toxicities of cell-free Hb could not be completely eliminated. A third generation, consisting of polymerised Hb encapsulated within larger particles, have been developed recently by several researchers, including our group.
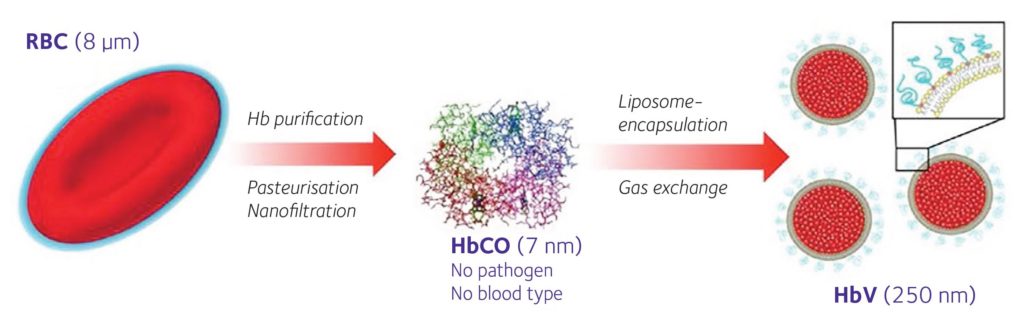
Our Hb vesicle (HbV) is a type of Hb encased in a liposomal capsule (Sakai, 2017) (Fig. 2). We believe it has several advantages as an artificial RBC, namely: it does not evoke a strong immune reaction; the scavenging of NO is minimal; it can be stored for long periods of time at room temperature; and to simplify the HbV production, we omitted the incorporation of carbonic anhydrase as transportation of carbon dioxide could likely be sufficiently handled by physical dissolution in plasma.
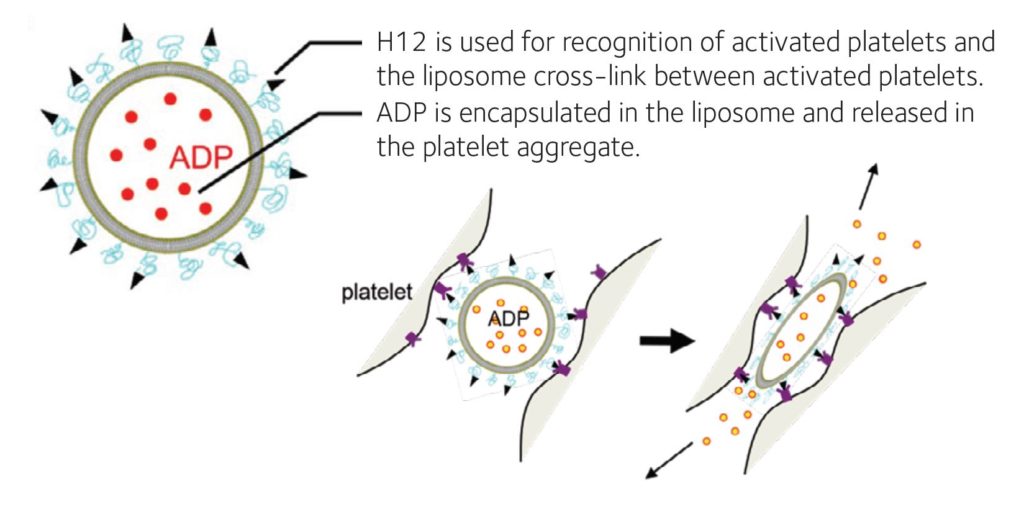
As for haemostasis, several researchers have also developed artificial platelets. Most are composed of small particles with surface modifications whereby the ligands for integrins on the activated platelets are added (Okamura et al., 2009; Hickman et al., 2018). Platelets made from induced pluripotent stem cells and adipose tissue-derived stem cells are also on the horizon. Our group has developed H12-(ADP)-liposomes (Okamura et al., 2009), which accumulate at a bleeding site through interaction with activated platelets via glycoprotein IIb/IIIa and H12, and augment platelet aggregation by releasing adenosine diphosphate (ADP) from the liposomes at the bleeding site for activation of platelets within a few minutes. H12-(ADP)-liposomes remained intact in the blood circulation for up to 24 hours after injection if they do not meet with activated platelets at the bleeding site. ADP released in blood is immediately metabolised to allantoin and fully discharged to urine within 6 hours (Fig. 3). These H12- (ADP)-liposomes can even be stored for at least 6 months at 4˚C without shaking while conventional platelet concentrate must be used within 4 days and requires constant shaking at 22˚C.
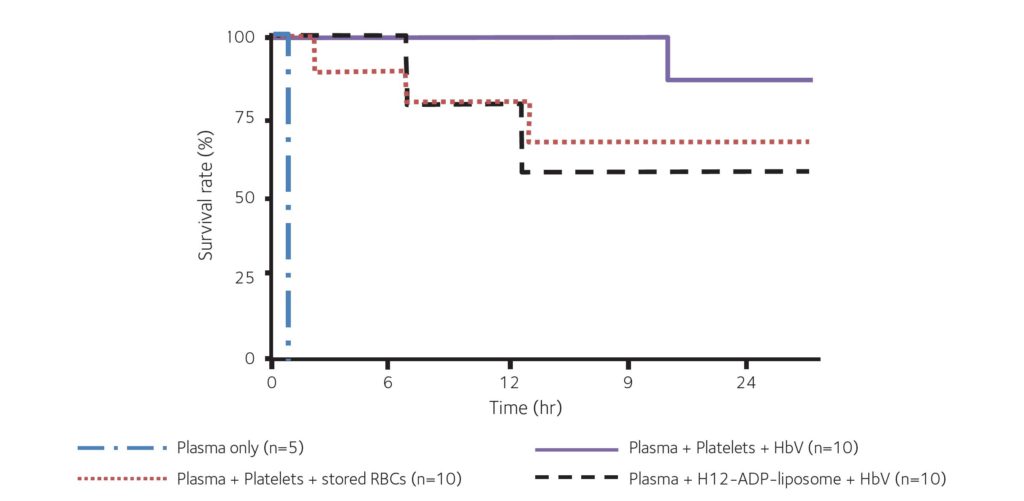
In our recent animal study, we demonstrated that, in combination with H12-(ADP)- liposomes, our HbVs helped to maintain both haemodynamics and haemostasis following severe traumatic haemorrhage with coagulopathy (Hagisawa et al., 2019). The outcomes were as good as that achieved with transfusion of allogenic RBCs and platelets, and this combination therapy drastically reduced mortality with no serious adverse effects observed (Fig. 4). We are pleased to announce that a phase 1 clinical study examining the safety of HbVs has just begun, and we look forward to the results.
New research also promises to advance this technology further. For example, the manufacturing of Hb encapsulated particles with carbonic anhydrase and superoxide dismutase/catalase to replicate the RBC function by maximising carbon dioxide transportation and free radical scavenging (Chang, 2017). Or mimetics like Erythromer, which better emulate RBC physiology, and also serve to inhibit methaemoglobin accumulation and NO sequestration (Pan et al., 2016), which is critical for long-term maintenance of haemoglobin oxygen-carrying capacity.
In conclusion, the early support of the coagulation system as well as oxygen delivery is essential for damage control following trauma. We consider artificial RBCs and artificial platelets an ideal substitute for component balanced transfusion and whole blood transfusion. We think that these blood substitutes will prove useful for prehospital resuscitation as they should achieve easy reserve, avoid immunological reactions, and do not require cross-matching of blood type.
References
Blundell J (1829). Observations on transfusion of blood by Dr. Blundell with a description of his Gravitator. Lancet 2, 321-324.
Chang TM (2017). Translational feasibility of soluble nanobiotherapeutics with enhanced red blood cell functions. Artificial Cells, Nanomedicine, and Biotechnology 45(4), 671-676. https://doi.org/10.1 080/21691401.2017.1293676.
Hall JE (2015). Guyton and Hall Textbook of Medical Physiology, 13th ed. Elsevier, Inc. p. 529.
Hagisawa K et al. (2019). Combination therapy using fibrinogen γ-chain peptide-coated, ADP-encapsulated liposomes and hemoglobin vesicles for trauma-induced massive hemorrhage in thrombocytopenic rabbits. Transfusion 59(10), 3186-3196. https://doi. org/10.1111/trf.15427.
Hickman DA et al. (2018). Intravenous synthetic platelet (SynthoPlate) nanoconstructs reduce bleeding and improve ‘golden hour’ survival in a porcine model of traumatic arterial hemorrhage. Scientific Reports 8, 3118. https://doi.org/10.1038/s41598-018- 21384-z.
Holcomb JB et al. (2015). Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. Journal of the American Medical Association 313(5), 471-482. https://doi:10.1001/jama.2015.12.
Okamura Y et al. (2009). Development of fibrinogen g-chain peptide-coated, adenosine diphosphate-encapsulated liposomes as a synthetic platelet substitute. Journal of Thrombosis and Haemostasis 7(3), 470-7. https://doi.org/10.1111/j.1538- 7836.2008.03269.x.
Pan D et al. (2016). Erythromer (EM), a nanoscale bio-synthetic artificial red cell: proof of concept and in vivo efficacy results. Blood 128 (22), 1027. https:// doi.org/10.1182/blood.V128.22.1027.1027.
Rahfeld P, Withers SG (2020). Toward universal donor blood: Enzymatic conversion of A and B to O type. Journal of Biological Chemistry 295(2), 325-334. https://doi.org/10.1074/jbc.REV119.008164.
Sakai H (2017). Overview of potential clinical applications of hemoglobin vesicles (HbV) as artificial red cells, evidenced by preclinical studies of the academic research consortium. Functional Materials 8(1), 10. https://doi.org/10.3390/jfb8010010.