Physiology News Magazine
Remote heart rhythm assessment by a smartphone camera:
How a mobile app made the difference during the COVID-19 pandemic
Features
Remote heart rhythm assessment by a smartphone camera:
How a mobile app made the difference during the COVID-19 pandemic
Features
Dominik Linz, Maastricht University Medical Centre and Cardiovascular Research Institute, Maastricht, Netherlands
Jeroen Hendriks, Flinders University and Royal Adelaide Hospital, Adelaide, Australia
https://doi.org/10.36866/pn.121.14
The Maastricht University Medical Center (MUMC+), is renowned for the management and treatment of patients with atrial fibrillation (AF). AF is the most prevalent cardiac arrhythmia. A progressive atrial remodelling process mainly characterised by atrial dilatation, cardiomyocyte hypertrophy and fibrosis, which creates a substrate to stabilise the arrhythmia. Additionally, ectopic discharges originating in the pulmonary veins can initiate and maintain AF episodes.
The treatment of AF patients involves the management of concomitant conditions such as hypertension, heart failure and obesity. Additionally, pharmacological treatment (by antiarrhythmic drugs) and catheter-guided electrical isolation of the pulmonary veins help to maintain sinus rhythm. To reduce ventricular rate during AF, beta-blockers, L-type calcium channel blockers and digoxin are used to control AF symptoms and to prevent tachycardia-associated complications.
AF is associated with morbidities such as heart failure and an increased risk of thromboembolic complications such as stroke, as well as mortality (Hindricks et al., 2020). Patients with AF are considered vulnerable and therefore monitoring of vital parameters, particularly heart rate and rhythm, is crucial to guide treatment decisions. A ventricular rate above 110 bpm during AF may lead to symptoms such as palpitations, dyspnoea, and may ultimately contribute to the development of heart failure (tachy-cardiomyopathy).
Under normal circumstances patients with AF will receive a face-to-face consultation in the outpatient clinic at the MUMC+. Prior to the consultation, an ECG is performed so that heart rate and rhythm information is available. During the coronavirus 2019 (COVID-19) pandemic, this was not possible. Social distancing was implemented to prevent extensive spread of the virus (or “flattening the curve”, as we’ve all come to know it). To protect our AF patients from being infected by COVID-19, face-to-face outpatient appointments were rapidly converted into teleconsultations, resulting in the proportion of patient contacts managed by teleconsultations increasing from fewer than 1% in 2019 to 76% in 2020. This meant that instead of seeing our patients with ECG information, we were calling them without any clinical data about heart rate and rhythm and it became clear that treatment decisions were going to be difficult.
Obtaining rate and rhythm information from patients at home
Prior to the COVID-19 pandemic, we were conducting some smaller research projects using mobile health (mHealth) technologies for remote heart rate and rhythm monitoring (Hermans et al., 2020; Hermans et al., 2021). Some of these mHealth devices are based on a single-lead ECG, whereas others base their heart rate and rhythm assessment on photoplethysmography (PPG) (Fig. 1) (Hermans et al., 2020).
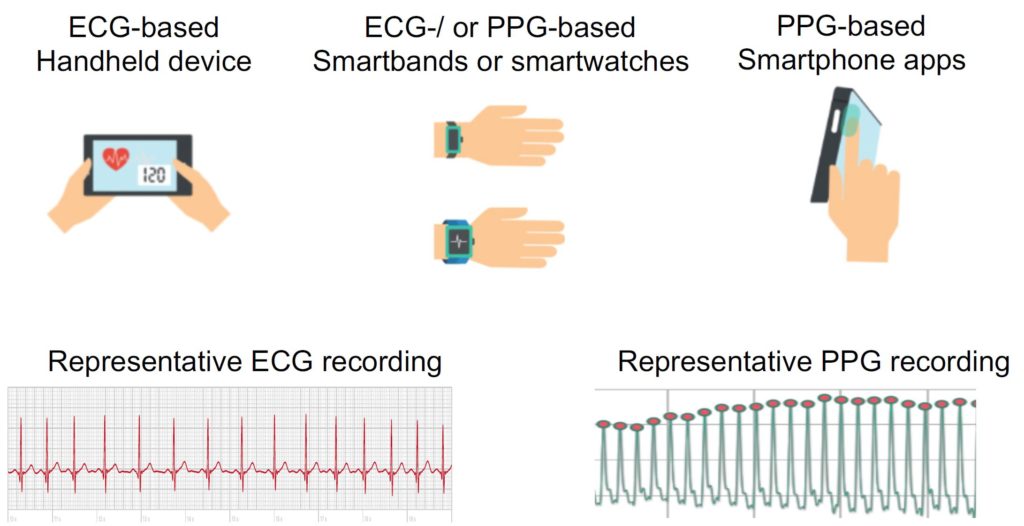
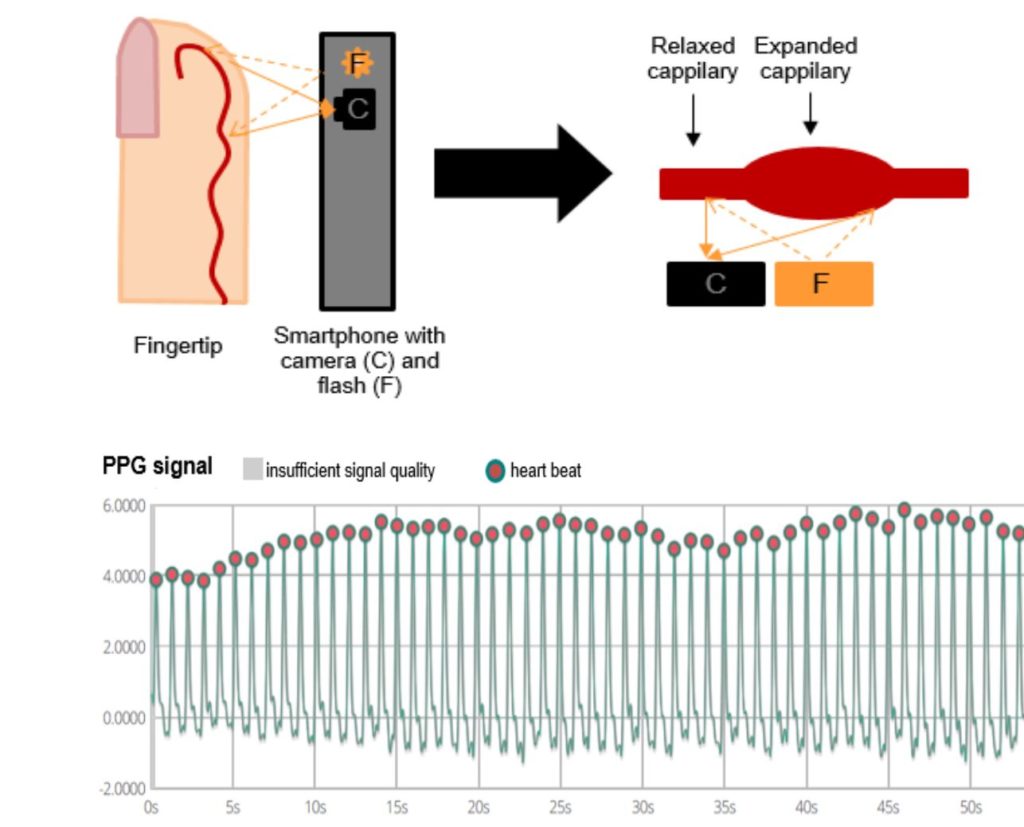
Traditionally, the cardiac electrical activity visualised by an ECG is the gold standard to assess the cardiac rhythm; however, emerging PPG technologies represent an alternative strategy. Clinically, PPG is currently used for finger pulse oximeters to measure oxygen saturation. Additionally, PPG can determine blood volume pulse variation in the local arterioles of the fingertip by measuring the amount of reflected light by a camera. There are applications available that convert the 60 Hz video data to raw signals. In this way, each individual pulse wave can be detected. The time intervals between consecutive pulse signals can be used to determine the heart rhythm and differentiate normal sinus rhythm from an arrhythmia such as AF (Fig. 2).
The differences in PPG and ECG technologies have important implications. Current AF guidelines of the European Society of Cardiology (ESC) state that ECG documentation, either on standard 12- lead ECG or on a single-lead ECG tracing, is required to establish the diagnosis of AF (Hindricks et al., 2020). Although PPG technology cannot be used to diagnose AF, it is able to accurately detect AF episodes. Several studies have been performed to determine the accuracy of PPG-based devices to detect ECG-confirmed AF. A meta-analysis by O’Sullivan et al. including four PPG-based mHealth devices presented an overall sensitivity and specificity of 94.2% and 95.8%, respectively (O’Sullivan et al., 2020). Therefore, PPG technology can be of great value in remotely assessing cardiac rates and rhythms to support management of patients who have been previously diagnosed by ECG with specific arrhythmias such as AF.
Additionally, the wide availability and affordability of PPG technologies (e.g. using smartphone camera) can be used in effective and economical AF screening programmes in patients with unknown AF to minimize the potential for harm in terms of inappropriate treatment (anticoagulation leading to an increased risk of major bleeding) and unnecessary investigations; maximising the diagnostic yield of AF that carries significant risk; and maximising the uptake of appropriate anticoagulation treatment in people with newly detected cases.
Our approach: TeleCheck-AF
While PPG technology has limitations for diagnosing AF, the wide accessibility and low cost of this technology – via smartphone apps using the built-in camera and flashlight of a mobile phone – made it an optimal tool for remote heart rate and rhythm monitoring of patients who have already been diagnosed with AF by ECG documentation. We literally turned smartphone technology, which is available to every individual, into a medical device to provide vital information to the clinicians required for remotely treating patients (Pluymaekers et al., 2020).
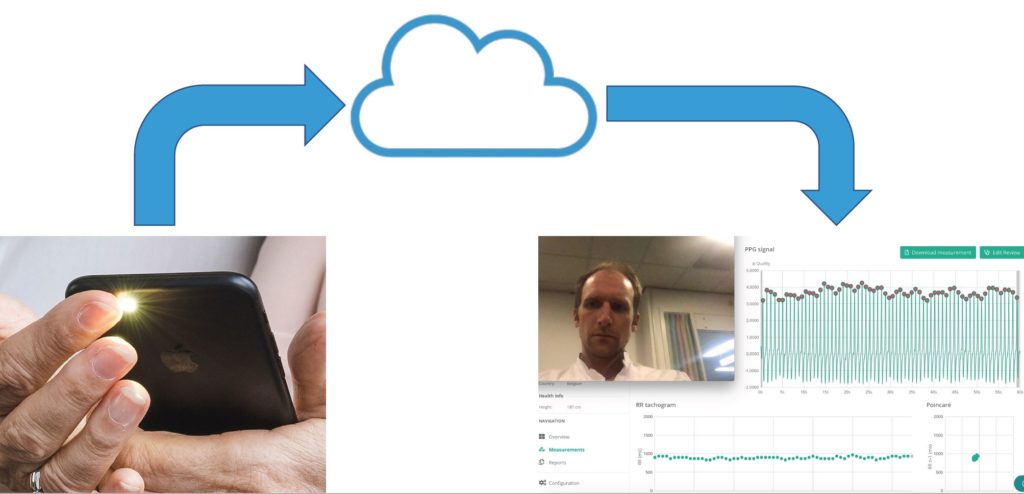
During the COVID-19 lockdown period, we contacted our AF patients 1 week prior to a scheduled teleconsultation and instructed them to download an app called FibriCheck (Conformitè Europëenne [CE]- marked and Food and Drug Administration [FDA]-approved), www.fibricheck.com). The patients were educated to perform three rate and rhythm recordings a day until the teleconsultation, and additional measures in case of symptoms. All recordings were instantly transmitted to a secured cloud system, which was accessible to the physician at the time of the teleconsultation (Fig. 3). We called this on-demand mHealth intervention: TeleCheck-AF (Pluymaekers et al., 2021; Linz et al., 2020).
TeleCheck-AF went viral during the COVID-19 pandemic
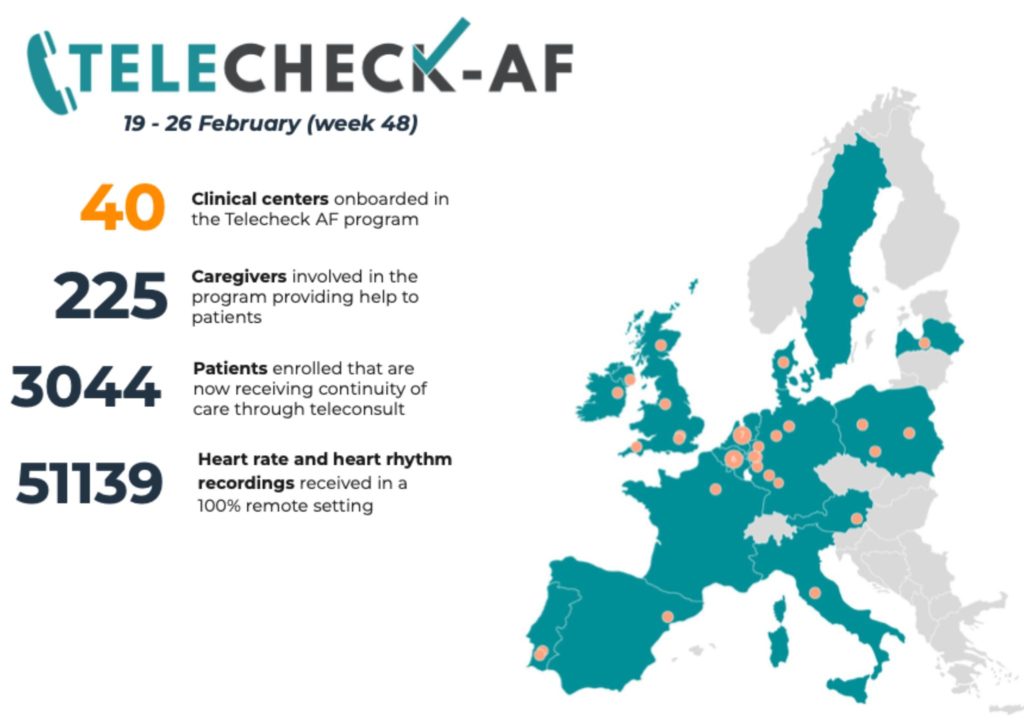
We started to communicate about our approach via Twitter (using the hashtag #TeleCheck-AF) and it was featured as an example of how to keep patients out of hospital using remote rhythm and rate monitoring by the European Society of Cardiology. We then implemented the TeleCheck-AF approach in numerous European centres during COVID-19. As a result of scaling up our activities, an additional 39 centres in 14 European countries were on-boarded to the TeleCheck-AF project. In total, 2,878 patients (including the patients in MUMC+) have been managed by the TeleCheck-AF approach outside the hospital remotely via teleconsultation (Fig. 4). This has reduced the burden for outpatient clinics and potentially reduced the risk to get infected by COVID-19 in a lot of patients. To assess how mHealth was used within the European TeleCheck-AF project, we are currently performing a retrospective analysis in most of the participating centres (Linz et al., 2020).
The next steps in remote AF management
According to current AF guidelines, AF management does not just involve rate and rhythm assessment. In addition to rate and rhythm control, the assessment and management of risk factors is also critical. Longitudinal monitoring of lifestyle factors and vital signs may impact how we manage our patients remotely in the future (Hermans et al., 2020; Gawalko et al., 2020). Already, smart wearable devices can capture important biometrics, which could help assess risk factors and lifestyle components longitudinally to initiate and guide risk-factor-modification programmes remotely.
Physical activity can be captured by using medical grade actigraphy via GPS and accelerometers to monitor the change in fitness (for example by www.fitbit.com). Lifestyle factors such as diet can be assessed in digital diaries (for example by www. Lumen.me or www.foodmarble.com). Some preliminary studies suggest that PPG signals recorded by mobile phones and watches can be calibrated against non-invasive blood pressure to monitor relative changes in blood pressure (www.mypblab.com). Additionally, several smartphone and smartwatch apps are starting to provide information about sleep patterns, quality and duration of sleep, by detecting variations in oxygen saturation and generating hypnographs (www.fitbit.com), as well as detecting interruptions in snoring patterns, which may be indicative of sleep apnoea (www.snorelab.com). Other devices or apps have been developed to capture and track biometrics related to breathing, such as measuring respiratory rate using impedance. Peripheral arterial tonometry (PAT) is currently used to detect respiratory events during sleep by measuring pulse oximetry desaturations. Also autonomic nervous system activation can be approximated. The quantification of sympathetic discharges can be assessed by a PAT amplitude reduction and concomitant increases in heart rate. These technologies may help to detect sleep-disordered breathing, an important modifiable risk factor for AF, in large patient populations in the future (Linz et al., 2018). Finally, hypoxaemia during daytime detected by oximetry is suggestive of lung disease such as chronic obstructive pulmonary disease, which is related to increased risk of AF and lower response to AF treatment strategies (Simons et al., 2021). Although several apps and wearables provide multiple biometrics that can be used for risk assessment and monitoring of treatment response, most apps are not CE-marked or FDA-approved and additional validation studies are required.
Clinical implementation and achieving digital equity
Smart wearable devices and smartphone apps used by our patients will provide more and more biometric information, which may guide our treatment decisions in the future. Despite the rapid progress of technology, there is still a significant gap between what we know and how we apply that knowledge in clinical practice. Therefore, it is crucial to invest in implementing this technology in existing clinical care pathways and apply that knowledge in the clinic. In addition to supporting the development of suitable mHealth infrastructures in the hospital, we also need to involve our patients in this process: a focus on education and empowerment to support self-care is crucial. The absence of reimbursement models and privacy considerations are the main reasons that mHealth use is currently largely patient-initiated. This leaves the patient with the difficult choice of deciding which device to use. Not all patients may be able to pay for the expensive devices or monthly fees to use a specific app. We as scientists, physicians and society need to make sure that the new technology is accessible and user-friendly to all patients, to ensure digital equity in mHealth and throughout modern medicine.
References
Gawałko M et al. (2020).A call for a more objective and longitudinal reporting of lifestyle components in cardiovascular research. International Journal of Cardiology Heart & Vasculature 27:100506. https://doi.org/10.1016/j.ijcha.2020.100506.
Hermans ANL et al. (2020). On-demand mobile health infrastructures to allow comprehensive remote atrial fibrillation and risk factor management through teleconsultation. Clinical Cardiology 43(11), 1232–39. https://doi.org/10.1002/clc.23469.
Hermans ANL et al. (2021). Long-term intermittent versus short continuous heart rhythm monitoring for the detection of atrial fibrillation recurrences after catheter ablation. International Journal of Cardiology. S0167-5273(20)34325-4. https://doi. org/10.1016/j.ijcard.2020.12.077.
Hindricks G et al. (2020) ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). European Heart Journal. https://doi.org/10.1093/ eurheartj/ehaa612.
Linz D et al. (2018). Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: A review. JAMA Cardiology 3(6), 532–40. https://doi.org/10.1001/ jamacardio.2018.0095.
Linz D et al. (2020). TeleCheck-AF for COVID-19. European Heart Journal 41(21), 1954–55. https:// doi.org/10.1093/eurheartj/ehaa404.
O’Sullivan JW et al. (2020). Accuracy of smartphone camera applications for detecting atrial fibrillation: a systematic review and meta-analysis. JAMA Network Open 3(4), e202064. https://doi.org/10.1001/ jamanetworkopen.2020.2064
Pluymaekers NAHA et al. (2020). On-demand app-based rate and rhythm monitoring to manage atrial fibrillation through teleconsultations during COVID-19. International Journal of Cardiology Heart & Vasculature. https://doi.org/10.1016/j. ijcha.2020.100533.
Pluymaekers NAHA et al. (2020). Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck- AF. EP Europace. https://doi.org/10.1093/europace/ euaa201.
Simons SO et al. (2021). Chronic obstructive pulmonary disease and atrial fibrillation: an interdisciplinary perspective. European Heart Journal. https://doi.org/10.1093/eurheartj/ehaa822.